
| Size | Price | Stock | Qty |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| Other Sizes |
Purity: ≥98%
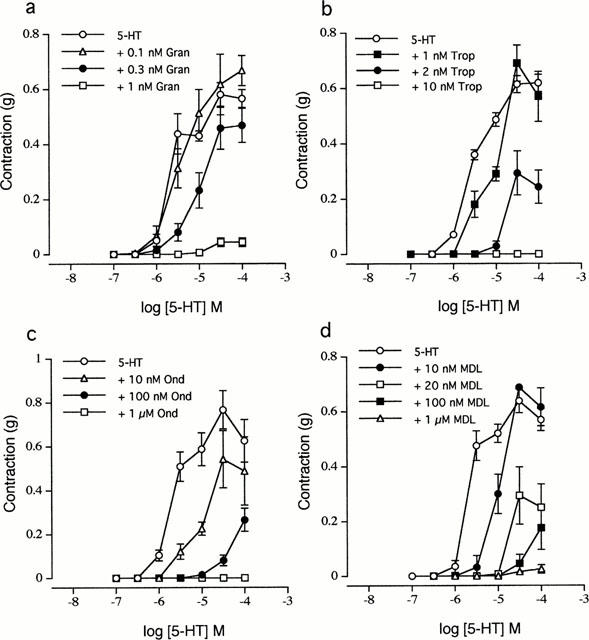
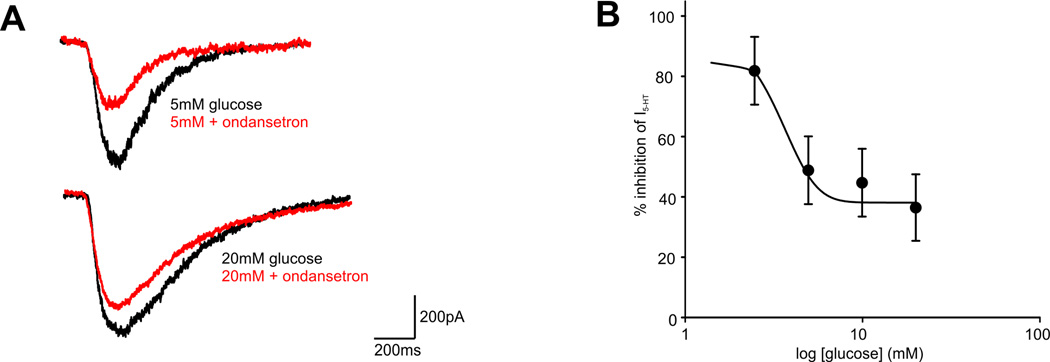
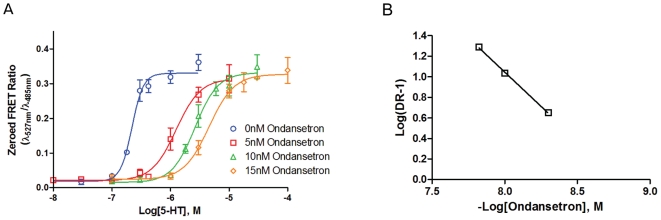
Ondansetron HCl (formerly GR 38032F, GR-C507/75; SN307; GR-C507/75; GRC-50775; GR-38032; SN-307; GR38032; Zofran) is an anti-emetic drug that acts as a potent serotonin 5-HT3 receptor antagonist. Its main purpose is to avoid nausea and vomiting that can result from radiation therapy and chemotherapy for cancer. At 0.3 nM, the 5-HT3A receptor antagonist ondansetron reversibly inhibited the 5-HT (30 microM) signal by 70%, and at 3 nM, it completely eliminated the response. Acute ondansetron administration improved auditory gating at 0.33 and 1 mg/kg, IP, but had no effect at the lowest tested dose of 0.1 mg/kg IP.
| Targets |
5-HT3 Receptor
|
||
|---|---|---|---|
| ln Vitro |
|
||
| ln Vivo |
|
||
| Cell Assay |
Ondansetron, an antagonist of the 5-HT3 receptor, inhibited the transient inward currents that 5-HT evoked (EC50 = 3.4 microM; Hill coefficient = 1.8) (IC50 = 103 pM). Ondansetron, a 5-HT3A receptor antagonist, reversibly inhibited the 5-HT (30 microM) signal by 70% at 0.3 nM and completely eliminated the response at 3 nM.
|
||
| Animal Protocol |
|
||
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Ondansetron is frequently used for nausea during and after cesarean section, usually in doses of 4 to 8 mg intravenously. Use during and after cesarean section appears to not affect the onset of breastfeeding. No adverse infant effects have been reported in this setting or among women who received ondansetron postpartum in a pharmacokinetic study. Use of ondansetron in nursing mothers beyond the immediate postpartum setting has not been studied well, but the drug is labeled for use in infants as young as 1 month of age. A computer model demonstrated that the amounts in milk are much less than this dose. No special precautions are required. ◉ Effects in Breastfed Infants In a pharmacokinetic study of 78 women who received ondansetron intravenously postpartum, no adverse effects were reported in their breastfed infants. ◉ Effects on Lactation and Breastmilk A randomized, double-blind study compared placebo to intravenous ondansetron 4 mg given after cesarean section as prophylaxis for postoperative nausea and vomiting. There was no difference in the time of the first breastfeeding between the two groups. In a retrospective study of women undergoing cesarean section deliveries, 3 regimens were compared: dexmedetomidine before anesthesia and during delivery (n = 115), normal saline before anesthesia and during delivery and dexmedetomidine after delivery (n = 109), and normal saline before anesthesia and during delivery (n = 168). All women received ondansetron 4 mg as needed and before removal of sutures. The average total amount of ondansetron consumed in the women ranged from 6 mg to 9 mg in the various groups. The time to first production of milk was similar in all groups (25 to 28 minutes). |
||
| References | |||
| Additional Infomation |
Ondansetron Hydrochloride is the hydrochloride salt of the racemic form of ondansetron, a carbazole derivative and a selective, competitive serotonin 5-hydroxytryptamine type 3 (5-HT3) receptor antagonist with antiemetic activity. Although its mechanism of action has not been fully characterized, ondansetron appears to competitively block the action of serotonin at 5HT3 receptors peripherally in the gastrointestinal tract as well as centrally in the area postrema of the CNS, where the chemoreceptor trigger zone (CTZ) for vomiting is located, resulting in the suppression of chemotherapy- and radiotherapy-induced nausea and vomiting.
A competitive serotonin type 3 receptor antagonist. It is effective in the treatment of nausea and vomiting caused by cytotoxic chemotherapy drugs, including cisplatin, and has reported anxiolytic and neuroleptic properties. See also: Ondansetron (has active moiety). Drug Indication Treatment of alcohol use disorder |
| Molecular Formula |
C18H19N3O
|
|---|---|
| Molecular Weight |
329.82
|
| Exact Mass |
329.129
|
| Elemental Analysis |
C, 65.55; H, 6.11; Cl, 10.75; N, 12.74; O, 4.85
|
| CAS # |
99614-01-4
|
| Related CAS # |
Ondansetron hydrochloride dihydrate; 103639-04-9; Ondansetron; 99614-02-5; Ondansetron-d3 hydrochloride; 1346605-02-4; Ondansetron-d6 hydrochloride; 1225442-22-7
|
| PubChem CID |
68647
|
| Appearance |
Solid powder
|
| Density |
1.27g/cm3
|
| Boiling Point |
546ºC at 760mmHg
|
| Melting Point |
178.5-179.5ºC
|
| Flash Point |
284ºC
|
| LogP |
3.93
|
| Hydrogen Bond Donor Count |
1
|
| Hydrogen Bond Acceptor Count |
2
|
| Rotatable Bond Count |
2
|
| Heavy Atom Count |
23
|
| Complexity |
440
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
O=C1C(CN2C=CN=C2C)CCC(N3C)=C1C4=C3C=CC=C4.[H]Cl
|
| InChi Key |
MKBLHFILKIKSQM-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C18H19N3O.ClH/c1-12-19-9-10-21(12)11-13-7-8-16-17(18(13)22)14-5-3-4-6-15(14)20(16)2;/h3-6,9-10,13H,7-8,11H2,1-2H3;1H
|
| Chemical Name |
9-methyl-3-[(2-methylimidazol-1-yl)methyl]-2,3-dihydro-1H-carbazol-4-one;hydrochloride
|
| Synonyms |
GR 38032F; GRC 50775; SN 307; GR-38032F; GRC-50775; SN-307; GR38032F; GRC50775; SN307; GR 38032F; trade name: Zofran
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples.
Injection Formulations
Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline)(e.g. IP/IV/IM/SC) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). View More
Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] Oral Formulations
Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). View More
Oral Formulation 3: Dissolved in PEG400 (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0320 mL | 15.1598 mL | 30.3196 mL | |
| 5 mM | 0.6064 mL | 3.0320 mL | 6.0639 mL | |
| 10 mM | 0.3032 mL | 1.5160 mL | 3.0320 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04169828 | Active Recruiting |
Drug: Methotrexate Drug: Ondansetron |
Juvenile Idiopathic Arthritis | University of British Columbia | August 2, 2019 | Not Applicable |
| NCT04209595 | Active Recruiting |
Drug: PLX038 Drug: Ondansetron |
Small Cell Lung Cancer Extra-Pulmonary Small Cell Carcinomas |
National Cancer Institute (NCI) |
April 8, 2020 | Phase 1 Phase 2 |
| NCT05378113 | Recruiting | Drug: Ondansetron Drug: Lidocaine |
Injection Site Irritation | Emory University | May 18, 2023 | Phase 2 |
| NCT05620641 | Recruiting | Drug: Ondansetron 8mg Drug: Gabapentin |
Sleeve Gastrectomy Morbid Obesity |
Tanta University | October 1, 2022 | Phase 3 |
| NCT03865290 | Recruiting | Drug: Ondansetron 8mg Drug: Placebo |
Indigestion Diabetes Mellitus |
Mayo Clinic | April 2, 2019 | Phase 2 |
 |
|---|
 |
 |