
| Size | Price | Stock | Qty |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
Purity: ≥98%
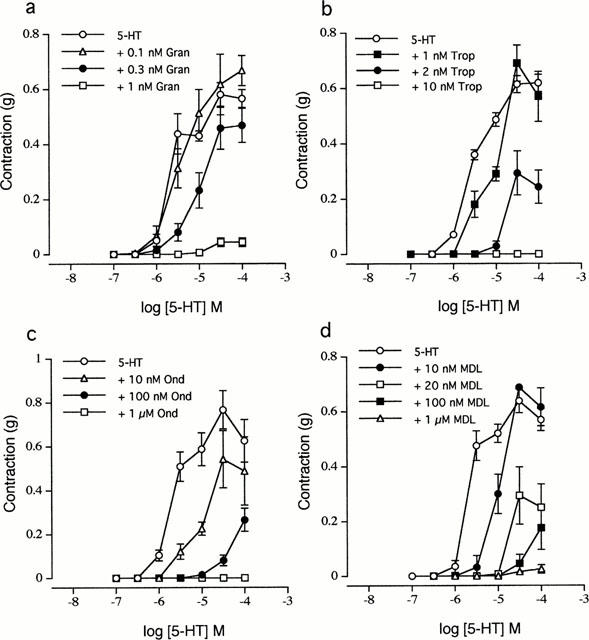
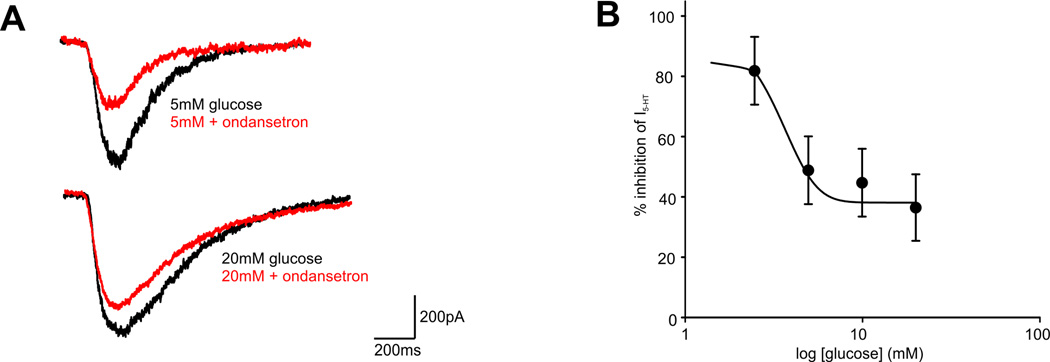
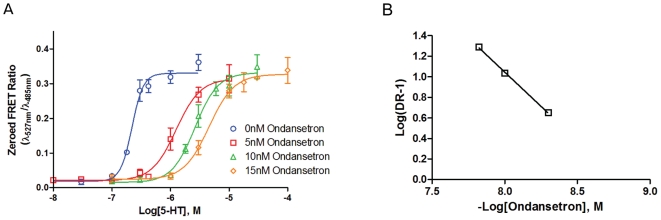
Ondansetron (GRC-50775; GR-38032; SN-307; GR38032; SN307; GR-C507/75; Zofran), an approved antiemetic drug, is a potent serotonin 5-HT3 receptor antagonist which is used to prevent nausea and vomiting caused by cancer chemotherapy, and radiation therapy. Ondansetron, a 5-HT3A receptor antagonist, reversibly inhibited the 5-HT (30 microM) signal by 70% at 0.3 nM and completely eliminated the response at 3 nM. The study found that auditory gating improved with 0.33 and 1 mg/kg, IP, but not with the lowest tested acute ondansetron dose of 0.1 mg/kg.
| Targets |
5-HT3
|
|---|---|
| ln Vivo |
Ondansetron (GR 38032; SN 307) (2.4-6 mg/kg; intraperitoneal injection; six times in 15 days) has a TD50 value of 3.7±0.6 mg/kg and an LD50 of 4.6±0.5 mg/kg in mice [4]. Ondansetron (8 mg/kg; intraperitoneal injection; once) combined with olanzapine has a good effect in preventing CINV in patients with NSCLC, especially for advanced patients [7]. Ondansetron (2 mg/kg; intraperitoneal injection; six consecutive days) Animal model: NSCLC patients receiving chemotherapy [7] Dosage: 8 mg Administration method: intraperitoneal injection (ip) Results: showed TD50 and LD50 doses of 3.7± 0.6 mg) exhibits anti-inflammatory effects through 5-HT3 receptors [8]. They are 4.6±0.5 mg/kg and 4.6±0.5 mg/kg respectively. Animal model: Male Swiss mice with colitis [8] Dosage: 2 mg/kg Administration: intraperitoneal injection (ip) Results: Demonstrated MPO activity and tumor necrosis factor-α, interleukin-6 and leukocyte-interleukin 1β was significantly reduced.
|
| Animal Protocol |
NSCLC Patients Treated With Chemotherapy
8 mg Intraperitoneal Injection (i.p.) |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
Ondansetron is absorbed from the gastrointestinal tract and undergoes some limited first-pass metabolism. Mean bioavailability in healthy subjects, following administration of a single 8-mg tablet, was recorded as being approximately 56% to 60%. Bioavailability is also slightly enhanced by the presence of food. Ondansetron systemic exposure does not increase proportionately to dose. The AUC from a 16-mg tablet was 24% greater than predicted from an 8-mg tablet dose. This may reflect some reduction of first-pass metabolism at higher oral doses. Following oral or IV administration, ondansetron is extensively metabolised and excreted in the urine and faeces. The volume of distribution of ondansetron has been recorded as being approximately 160L. The clearance values determined for ondansetron in various patient age groups were recorded as approximately 0.38 L/h/kg in normal adult volunteers aged 19-40 yrs, 0.32 L/h/kg in normal adult volunteers aged 61-74 yrs, 0.26 L/h/kg in normal adult volunteers aged >=75 yrs. Ondansetron is a 5-HT3 receptor antagonist that is an effective anti-emetic in cats. The purpose of this study was to evaluate the pharmacokinetics of ondansetron in healthy cats. Six cats with normal complete blood count, serum biochemistry, and urinalysis received 2 mg oral (mean 0.43 mg/kg), subcutaneous (mean 0.4 mg/kg), and intravenous (mean 0.4 mg/kg) ondansetron in a cross-over manner with a 5-day wash out. Serum was collected prior to, and at 0.25, 0.5, 1, 2, 4, 8, 12, 18, and 24 hr after administration of ondansetron. Ondansetron concentrations were measured using liquid chromatography coupled to tandem mass spectrometry. Noncompartmental pharmacokinetic modeling and dose interval modeling were performed. Repeated measures anova was used to compare parameters between administration routes. Bioavailability of ondansetron was 32% (oral) and 75% (subcutaneous). Calculated elimination half-life of ondansetron was 1.84 + or - 0.58 hr (intravenous), 1.18 + or - 0.27 hr (oral) and 3.17 + or - 0.53 hr (subcutaneous). The calculated elimination half-life of subcutaneous ondansetron was significantly longer (P < 0.05) than oral or intravenous administration. Subcutaneous administration of ondansetron to healthy cats is more bioavailable and results in a more prolonged exposure than oral administration. This information will aid management of emesis in feline patients. Nausea and vomiting are some of the major side effects caused by certain drug therapies, e.g. chemotherapy, radiotherapy and general anesthesia. Because of the nature of the symptoms, oral delivery is inappropriate, while intravenous administration may be unpractical. The aim of the present study was to develop a transdermal gel (2% Klucel) for ondansetron, a first line 5-HT3-receptor-antagonist antiemetic. The effects of the penetration enhancer camphor and isopropyl-myristate (IPM) were first investigated in-vitro using modified Franz diffusion-cells and then tested in-vivo in a rabbit model by measuring skin and plasma concentrations. Since a disadvantage of transdermal delivery is a prolonged lag-time, the effect of skin treatment with a micro-needle roller was tested. The in-vitro permeation studies through excised porcine ear skin showed that the presence of 2.5% camphor or IPM increased steady state flux by 1.2- and 2.5-fold, respectively, compared to the control gel. Ondansetron was not detectable in either skin or plasma following in-vivo application of the base-gel, whereas the camphor gel and IPM gel delivered 20 and 81 ug/sq cm of ondansetron, respectively. Microporation led to an increase in plasma Cmax and AUC by 10.47 + or - 1.68-fold and 9.31 + or - 4.91-fold, respectively, for the camphor gel, and by 2.31 + or -0.53-fold and 1.59 + or - 0.38-fold, respectively for the IPM gel. In conclusion, the 2.5% IPM gel demonstrated optimal in-vivo transdermal flux. Skin pretreatment with a micro-needle roller slightly improved the delivery of the IPM gel, whereas dramatically increased the transdermal delivery of the camphor gel. Ondansetron is a potent antiemetic drug that has been commonly used to treat acute and chemotherapy-induced nausea and vomiting (CINV) in dogs. The aim of this study was to perform a pharmacokinetic analysis of ondansetron in dogs following oral administration of a single dose. A single 8-mg oral dose of ondansetron was administered to beagles (n = 18), and the plasma concentrations of ondansetron were measured by liquid chromatography-tandem mass spectrometry. The data were analyzed by modeling approaches using ADAPT5, and model discrimination was determined by the likelihood-ratio test. The peak plasma concentration (Cmax ) was 11.5 +/- 10.0 ng/mL at 1.1 +/- 0.8 hr. The area under the plasma concentration vs. time curve from time zero to the last measurable concentration was 15.9 +/- 14.7 ng hr/mL, and the half-life calculated from the terminal phase was 1.3 +/- 0.7 hr. The interindividual variability of the pharmacokinetic parameters was high (coefficient of variation > 44.1%), and the one-compartment model described the pharmacokinetics of ondansetron well. The estimated plasma concentration range of the usual empirical dose from the Monte Carlo simulation was 0.1-13.2 ng/mL. These findings will facilitate determination of the optimal dose regimen for dogs with CINV. Ondansetron is the drug of choice to prevent nausea in women undergoing cesarean surgery and can be used to prevent neonatal abstinence syndrome (NAS). The pharmacokinetics of ondansetron have not been characterized in pregnant women or in newborns. A nonlinear mixed-effects modeling approach was used to analyze plasma samples obtained from 20 nonpregnant and 40 pregnant women following a single administration of 4 or 8 mg ondansetron, from umbilical cord blood at delivery, and from neonates after birth. The analysis indicates that: ondansetron disposition is not affected by pregnancy (P > 0.05), but influenced by dose (P < 0.05), and is characterized by rapid transplacental transfer and longer elimination half-life in neonates compared to their mother. A dosing regimen for prevention of NAS was designed based on the model. The regimen involves IV administration of 4 mg to the mothers shortly before cord clamping, or oral administration of 0.07 mg/kg (or equivalently 0.04 mg/kg IV) to neonates. For more Absorption, Distribution and Excretion (Complete) data for Ondansetron (8 total), please visit the HSDB record page. Metabolism / Metabolites In vitro metabolism studies have shown that ondansetron is a substrate for human hepatic cytochrome P450 enzymes, including CYP1A2, CYP2D6 and CYP3A4. In terms of overall ondansetron turnover, CYP3A4 played the predominant role. Because of the multiplicity of metabolic enzymes capable of metabolizing ondansetron, it is likely that inhibition or loss of one enzyme (e.g. CYP2D6 enzyme deficiency) will be compensated by others and may result in little change in overall rates of ondansetron clearance. Following oral or IV administration, ondansetron is extensively metabolised and excreted in the urine and faeces. In humans, less than 10% of the dose is excreted unchanged in the urine. The major urinary metabolites are glucuronide conjugates (45%), sulphate conjugates (20%) and hydroxylation products (10%). The primary metabolic pathway is subsequently hydroxylation on the indole ring followed by subsequent glucuronide or sulfate conjugation. Although some nonconjugated metabolites have pharmacologic activity, these are not found in plasma at concentrations likely to significantly contribute to the biological activity of ondansetron. Ondansetron is extensively metabolized in humans, with approximately 5% of a radiolabeled dose recovered as the parent compound from the urine. The primary metabolic pathway is hydroxylation on the indole ring followed by subsequent glucuronide or sulfate conjugation. Although some nonconjugated metabolites have pharmacologic activity, these are not found in plasma at concentrations likely to significantly contribute to the biological activity of ondansetron. Ondansetron has known human metabolites that include 6-hydroxy-ondansetron, 7-hydroxy-ondansetron, and 8-hydroxy-ondansetron. Hepatic Half Life: 5.7 hours Biological Half-Life The half-life of ondansetron after either an 8 mg oral dose or intravenous dose was approximately 3-4 hours and could be extended to 6-8 hours in the elderly. In humans ... elimination half lives are approximately 3-4 hours, but are prolonged in elderly patients. ... Six cats with normal complete blood count, serum biochemistry, and urinalysis received 2 mg oral (mean 0.43 mg/kg), subcutaneous (mean 0.4 mg/kg), and intravenous (mean 0.4 mg/kg) ondansetron in a cross-over manner with a 5-day wash out. Serum was collected prior to, and at 0.25, 0.5, 1, 2, 4, 8, 12, 18, and 24 hr after administration of ondansetron. ... Calculated elimination half-life of ondansetron was 1.84 + or - 0.58 hr (intravenous), 1.18 + or - 0.27 hr (oral) and 3.17 + or - 0.53 hr (subcutaneous). The calculated elimination half-life of subcutaneous ondansetron was significantly longer (P < 0.05) than oral or intravenous administration. ... ... A single 8-mg oral dose of ondansetron was administered to beagles (n = 18), ... and the half-life calculated from the terminal phase was 1.3 +/- 0.7 hr. ... |
| Toxicity/Toxicokinetics |
Toxicity Summary
IDENTIFICATION AND USE: Ondansetron forms as crystals from methanol. It is a drug used for the prevention of nausea and vomiting associated with highly emetogenic cancer chemotherapy in both human and veterinary cases. Prolongation of the QT interval and cases of torsades de pointes have been reported in patients receiving ondansetron. Liver failure and death have been reported rarely in patients with cancer receiving ondansetron concomitantly with other drugs, including potentially hepatotoxic cytotoxic chemotherapy and antibiotics. Ondansetron hydrochloride may cause a serious anaphylactic reaction. In a study of children whose mothers received promethazine or ondansetron during pregnancy, no clinically significant adverse neurobehavioral effects or obstetric outcomes were identified. According to a different study, the teratogenic risk with ondansetron is low but an increased risk for a cardiac septum defect is likely. There was also no evidence of damage to genetic material noted in in vitro chromosome aberration tests using human peripheral lymphocytes. ANIMAL STUDIES: Ondansetron was administered orally to rats at doses of 1, 4, and 15 mg/kg during gametogenesis, mating, pregnancy, and lactation periods. It is proposed that the maximum noneffective dose of ondansetron was 4 and 15 mg/kg with respect to general toxicity and reproductive capacity in F0 animals respectively. The maximum noneffective dose with respect to development in F1 and F2 animals was suggested to be 15 mg/kg. After IV administration of 0.5, 1.5, and 4 mg/kg of ondansetron daily during gestation and lactation period, the results suggest that the maximum noneffective dose of ondansetron for general toxicity in dams was 1.5 mg/kg and that for reproduction toxicity in dams and developmental toxicity in fetuses and offspring was 4 mg/kg. A slight maternal toxicity was observed at the highest dose level in intravenous organogenesis (4.0 mg/kg/day) studies in the rabbit. Effects included maternal body weight loss and increased incidence of early fetal death. There was no evidence of damage to genetic material noted in in vitro V-79 mammalian cell mutation studies or in vivo chromosome aberration assays in mouse bone marrow. No evidence of mutagenicity was observed in microbial mutagen tests using mutant strains of Salmonella typhimurium, Escherichia coli or Saccharomyces cerevisiae, with or without a rat liver post-mitochondrial metabolizing system. Carcinogenic effects were not seen in 2-year studies in rats and mice with oral ondansetron doses up to 10 and 30 mg/kg/day, respectively. Ondansetron is a selective serotonin 5-HT3 receptor antagonist. The antiemetic activity of the drug is brought about through the inhibition of 5-HT3 receptors present both centrally (medullary chemoreceptor zone) and peripherally (GI tract). This inhibition of 5-HT3 receptors in turn inhibits the visceral afferent stimulation of the vomiting center, likely indirectly at the level of the area postrema, as well as through direct inhibition of serotonin activity within the area postrema and the chemoreceptor trigger zone. Interactions The nephrotoxicity limits the clinical application of cisplatin. Human organic cation transporter 2 (OCT2) and multidrug and toxin extrusion proteins (MATEs) work in concert in the elimination of cationic drugs such as cisplatin from the kidney. We hypothesized that co-administration of ondansetron would have an effect on cisplatin nephrotoxicity by altering the function of cisplatin transporters. The inhibitory potencies of ondansetron on metformin accumulation mediated by OCT2 and MATEs were determined in the stable HEK-293 cells expressing these transporters. The effects of ondansetron on drug disposition in vivo were examined by conducting the pharmacokinetics of metformin, a classical substrate for OCTs and MATEs, in wild-type and Mate1-/- mice. The nephrotoxicity was assessed in the wild-type and Mate1-/- mice received cisplatin with and without ondansetron. Both MATEs, including human MATE1, human MATE2-K, and mouse Mate1, and OCT2 (human and mouse) were subject to ondansetron inhibition, with much greater potencies by ondansetron on MATEs. Ondansetron significantly increased tissue accumulation and pharmacokinetic exposure of metformin in wild-type but not in Mate1-/- mice. Moreover, ondansetron treatment significantly enhanced renal accumulation of cisplatin and cisplatin-induced nephrotoxicity which were indicated by increased levels of biochemical and molecular biomarkers and more severe pathohistological changes in mice. Similar increases in nephrotoxicity were caused by genetic deficiency of MATE function in mice. Therefore, the potent inhibition of MATEs by ondansetron enhances the nephrotoxicity associated with cisplatin treatment in mice. Potential nephrotoxic effects of combining the chemotherapeutic cisplatin and the antiemetic 5-hydroxytryptamine-3 (5-HT3) receptor antagonists, such as ondansetron, should be investigated in patients. Although no pharmacokinetic drug interaction between ondansetron and tramadol has been observed, data from 2 small trials indicate that ondansetron may be associated with an increase in patient controlled administration of tramadol. Serotonin syndrome (including altered mental status, autonomic instability, and neuromuscular abnormalities) has been described following the concomitant use of 5-HT3 receptor antagonists and other serotonergic drugs, including selective serotonin reuptake inhibitors (SSRIs) and serotonin and noradrenaline reuptake inhibitors. In patients treated with potent inducers of CYP3A4 (i.e., phenytoin, carbamazepine, and rifampicin), the clearance of ondansetron was significantly increased and ondansetron blood concentrations were decreased. However, on the basis of available data, no dosage adjustment for ondansetron is recommended for patients on these drugs. For more Interactions (Complete) data for Ondansetron (8 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat IV 15-20 mg/kg LD50 Rat oral 100-150 mg/kg LD50 Mouse IV 1.0-2.5 mg/kg LD50 Mouse oral 10-30 mg/kg |
| References |
|
| Additional Infomation |
Therapeutic Uses
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Ondansetron is included in the database. Prevention of nausea and vomiting associated with highly emetogenic cancer chemotherapy, including cisplatin ... . /Included in US product label/ Prevention of nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy. /Included in US product label/ Prevention of nausea and vomiting associated with radiotherapy in patients receiving either total body irradiation, single high-dose fraction to the abdomen, or daily fractions to the abdomen. /Included in US product label/ For more Therapeutic Uses (Complete) data for Ondansetron (7 total), please visit the HSDB record page. Drug Warnings Because of the risk of QT-interval prolongation, ondansetron should be avoided in patients with congenital long QT syndrome. ECG monitoring is recommended in patients with electrolyte abnormalities such as hypokalemia or hypomagnesemia, congestive heart failure, or bradyarrhythmias and in those receiving other drugs known to prolong the QT interval. Electrolyte abnormalities should be corrected prior to IV administration of ondansetron. Because effects of ondansetron on the QT interval are dose related, use of single IV doses exceeding 16 mg should be avoided. Patients receiving ondansetron should be advised to seek immediate medical care if feelings of faintness, lightheadedness, irregular heartbeat, shortness of breath, or dizziness occur. Based on reports of profound hypotension and loss of consciousness when apomorphine was administered with ondansetron, concomitant use of apomorphine with ondansetron is contraindicated Advise patients of the possibility of serotonin syndrome with concomitant use of Zofran and another serotonergic agent such as medications to treat depression and migraines. Advise patients to seek immediate medical attention if the following symptoms occur: changes in mental status, autonomic instability, neuromuscular symptoms with or without gastrointestinal symptoms. Seizures (including tonic-clonic seizures) have been reported rarely in patients receiving ondansetron. For more Drug Warnings (Complete) data for Ondansetron (32 total), please visit the HSDB record page. Pharmacodynamics Ondansetron is a highly specific and selective serotonin 5-HT3 receptor antagonist, not shown to have activity at other known serotonin receptors and with low affinity for dopamine receptors,. The serotonin 5-HT3 receptors are located on the nerve terminals of the vagus in the periphery, and centrally in the chemoreceptor trigger zone of the area postrema,. The temporal relationship between the emetogenic action of emetogenic drugs and the release of serotonin, as well as the efficacy of antiemetic agents, suggest that chemotherapeutic agents release serotonin from the enterochromaffin cells of the small intestine by causing degenerative changes in the GI tract,. The serotonin then stimulates the vagal and splanchnic nerve receptors that project to the medullary vomiting center, as well as the 5-HT3 receptors in the area postrema, thus initiating the vomiting reflex, causing nausea and vomiting,. Moreover, the effect of ondansetron on the QTc interval was evaluated in a double-blind, randomized, placebo and positive (moxifloxacin) controlled, crossover study in 58 healthy adult men and women. Ondansetron was tested at single doses of 8 mg and 32 mg infused intravenously over 15 minutes. At the highest tested dose of 32 mg, prolongation of the Fridericia-corrected QTc interval (QT/RR0.33=QTcF) was observed from 15 min to 4 h after the start of the 15 min infusion, with a maximum mean (upper limit of 90% CI) difference in QTcF from placebo after baseline-correction of 19.6 (21.5) msec at 20 min. At the lower tested dose of 8 mg, QTc prolongation was observed from 15 min to 1 h after the start of the 15-minute infusion, with a maximum mean (upper limit of 90% CI) difference in QTcF from placebo after baseline-correction of 5.8 (7.8) msec at 15 min. The magnitude of QTc prolongation with ondansetron is expected to be greater if the infusion rate is faster than 15 minutes. The 32 mg intravenous dose of ondansetron must not be administered. No treatment-related effects on the QRS duration or the PR interval were observed at either the 8 or 32 mg dose. An ECG assessment study has not been performed for orally administered ondansetron. On the basis of pharmacokinetic-pharmacodynamic modelling, an 8 mg oral dose of ondansetron is predicted to cause a mean QTcF increase of 0.7 ms (90% CI -2.1, 3.3) at steady-state, assuming a mean maximal plasma concentration of 24.7 ng/mL (95% CI 21.1, 29.0). The magnitude of QTc prolongation at the recommended 5 mg/m2 dose in pediatrics has not been studied, but pharmacokinetic-pharmacodynamic modeling predicts a mean increase of 6.6 ms (90% CI 2.8, 10.7) at maximal plasma concentrations. In healthy subjects, single intravenous doses of 0.15 mg/kg of ondansetron had no effect on esophageal motility, gastric motility, lower esophageal sphincter pressure, or small intestinal transit time. Multiday administration of ondansetron has been shown to slow colonic transit in healthy subjects. Ondansetron has no effect on plasma prolactin concentrations. |
| Molecular Formula |
C18H19N3O
|
|---|---|
| Molecular Weight |
293.36
|
| Exact Mass |
293.152
|
| Elemental Analysis |
C, 73.69; H, 6.53; N, 14.32; O, 5.45
|
| CAS # |
99614-02-5
|
| Related CAS # |
Ondansetron hydrochloride dihydrate; 103639-04-9; Ondansetron-d5; 1219798-86-3; Ondansetron hydrochloride; 99614-01-4; Ondansetron-d3; 1132757-82-4; Ondansetron-13C,d3; 2699607-85-5
|
| PubChem CID |
4595
|
| Appearance |
White to off-white solid powder
|
| Density |
1.3±0.1 g/cm3
|
| Boiling Point |
546.0±30.0 °C at 760 mmHg
|
| Melting Point |
231 - 232ºC
|
| Flash Point |
284.0±24.6 °C
|
| Vapour Pressure |
0.0±1.5 mmHg at 25°C
|
| Index of Refraction |
1.678
|
| LogP |
2.07
|
| Hydrogen Bond Donor Count |
0
|
| Hydrogen Bond Acceptor Count |
2
|
| Rotatable Bond Count |
2
|
| Heavy Atom Count |
22
|
| Complexity |
440
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
O=C1C2C3=C([H])C([H])=C([H])C([H])=C3N(C([H])([H])[H])C=2C([H])([H])C([H])([H])C1([H])C([H])([H])N1C([H])=C([H])N=C1C([H])([H])[H]
|
| InChi Key |
FELGMEQIXOGIFQ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C18H19N3O/c1-12-19-9-10-21(12)11-13-7-8-16-17(18(13)22)14-5-3-4-6-15(14)20(16)2/h3-6,9-10,13H,7-8,11H2,1-2H3
|
| Chemical Name |
9-methyl-3-[(2-methylimidazol-1-yl)methyl]-2,3-dihydro-1H-carbazol-4-one
|
| Synonyms |
GR 38032; SN 307; GR 38032F; GRC50775; GR-38032; SN-307; GR38032; SN307; GR-C507/75; trade name: Zofran
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: This product requires protection from light (avoid light exposure) during transportation and storage. |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 1 mg/mL (3.41 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 10.0 mg/mL clear DMSO stock solution to 400 μL of PEG300 and mix evenly; then add 50 μL of Tween-80 to the above solution and mix evenly; then add 450 μL of normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 1 mg/mL (3.41 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 10.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 1 mg/mL (3.41 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.4088 mL | 17.0439 mL | 34.0878 mL | |
| 5 mM | 0.6818 mL | 3.4088 mL | 6.8176 mL | |
| 10 mM | 0.3409 mL | 1.7044 mL | 3.4088 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
Antiemetic Fosaprepitant To Remedy Nausea and Vomiting
CTID: NCT06382012
Phase: Phase 2/Phase 3 Status: Recruiting
Date: 2024-11-12
 |
|---|
 |
 |