
| Size | Price | Stock | Qty |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
Purity: =99.37%
Doxorubicin (Adriamycin; NSC-123127; FI-106; Adriblastine; Adrimedac) is a naturally occuring anthracycline antibiotic isolated from the bacterium Streptomyces peucetius var. caesius with potent anticancer activity and was approved as an anticancer chemotherapeutic medication. This is an inhibitor of DNA topoisomerase II that can cause apoptosis and damage to DNA in tumor cells. Danunorubicin's hydroxylated congener is called doxorubicin. In order to stop DNA replication and ultimately stop protein synthesis, doxorubicin works by intercalating between base pairs in the DNA helix. Doxorubicin also inhibits the enzyme topoisomerase II.
| Targets |
Topoisomerase I ( IC50 = 0.8 μM ); Topoisomerase II ( IC50 = 2.67 μM ); Daunorubicins/Doxorubicins; HIV-1; - DNA topoisomerase II (inhibits enzyme activity by stabilizing the cleavable complex) [1]
|
|---|---|
| ln Vitro |
In vitro activity: Doxorubicin, an antibiotic anthracycline, is widely believed to exhibit its anti-tumor activity on two basic levels: it modifies DNA and generates free radicals to cause DNA damage that causes cancer cells to undergo apoptosis. Doxorubicin inhibits DNA topoisomerase II (TOP2) and can intercalate into DNA strands to prevent DNA synthesis. When cells are multiplying quickly and expressing a lot of TOP2, doxorubicin works best. Additionally, doxorubicin can cause apoptosis by releasing cytochrome c from the mitochondria, ceramide (which activates p53 or other downstream pathways like JNK), the degradation of Akt by serine threonine proteases, an increase in the production of FasL (death receptor Fas/CD95 ligand) mRNA, and an increase in free radical production. The doxorubicin-resistant breast cancer cell line MCF7/Dx exhibits suppression of resistance upon pre-treatment with GSNO (nitrosoglutathione), which is accompanied by increased protein glutathionylation and doxorubicin accumulation in the nucleus. Elevated cyclin G2 (CycG2) expression and protein phosphorylation in the ATM, ATM, and Rad3-related (ATR) signaling pathways are responsible for doxorubicin-induced G2/M checkpoint arrest. In mouse embryonic fibroblasts (MEFs) and cardiomyocytes, doxorubicin inhibits AMP-activated protein kinase (AMPK), leading to SIRT1 dysfunction, p53 accumulation, and increased cell death. These effects can be further sensitized by pre-inhibition of AMPK. Doxorubicin causes a noticeable heat shock response, and in neuroblastoma cells, it increases the apoptotic effect by either inhibiting or silencing heat shock proteins. When administered in nanomolar doses to neuroblastoma cells, doxorubicin causes a dose-dependent over-ubiquitination of a particular set of proteins without any detectable proteasome inhibition. It also causes a decrease in the activity of ubiquitinated enzymes like lactate dehydrogenase and α-enolase, whose protein ubiquitination patterns resemble those of the proteasome inhibitor bortezomib, suggesting that Doxorubicin may also cause protein damage.
- Doxorubicin inhibits DNA topoisomerase II by stabilizing the enzyme-DNA cleavable complex, leading to DNA double-strand breaks and subsequent cell cycle arrest and apoptosis. It shows cytotoxic activity against various cancer cell lines, with potency varying by cell type [1] - Doxorubicin inhibits human DNA topoisomerase I with an IC50 of 16 μM, as measured by the relaxation of supercoiled DNA. This inhibition is weaker compared to its effect on topoisomerase II [3] - In combination with simvastatin, Doxorubicin exhibits synergistic cytotoxicity against human cancer cell lines (MCF-7, HT-29, HepG2). The combination reduces cell viability more effectively than either agent alone, with the synergistic effect observed at various concentration ratios [4] - Doxorubicin enhances the cytotoxicity of Apo2L/TRAIL in prostate cancer cell lines (PC-3, DU145). Co-treatment increases apoptosis, as indicated by increased caspase-3 activity and annexin V staining, compared to either agent alone [5] - A doxorubicin-conjugated anti-HIV-1 envelope antibody inhibits HIV-1 replication in vitro, reducing viral p24 antigen levels in infected T cells. The conjugate selectively targets infected cells expressing the viral envelope protein, minimizing toxicity to uninfected cells [7] |
| ln Vivo |
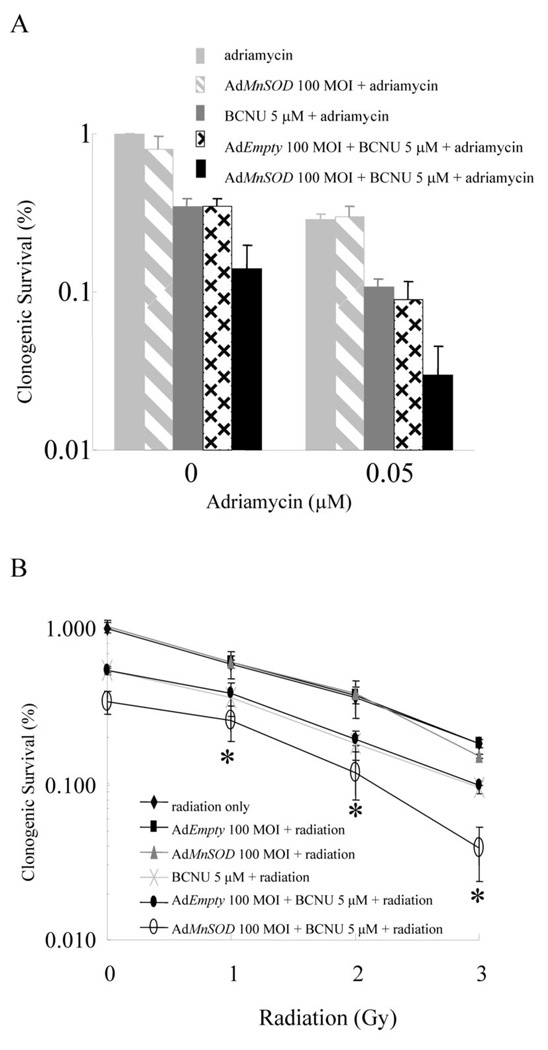
The most effective method for reducing the size of MB231 tumors and extending the survival of mice in vivo is to combine doxorubicin with adenoviral MnSOD (AdMnSOD) and 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU). Doxorubicin is indispensable in the treatment of solid tumors in childhood, soft tissue sarcomas, osteosarcomas, Kaposi's sarcoma, oesophageal carcinomas, Hodgkin and non-Hodgkin lymphomas, and solid tumors of the breast and esophagus. However, its use is restricted by the toxic side effects, both acute and chronic.
- In a prostate cancer xenograft model (PC-3 cells implanted in nude mice), combination treatment with Doxorubicin (2 mg/kg, intraperitoneal, once weekly) and Apo2L/TRAIL (15 mg/kg, intravenous, twice weekly) significantly reduces tumor growth compared to either agent alone. The combination also prolongs survival without increasing systemic toxicity [5] - In rats, Doxorubicin induces cardiotoxicity characterized by left ventricular dysfunction, increased serum cardiac troponin I levels, and histopathological changes (myocardial vacuolization, fibrosis). These effects are dose-dependent, with higher cumulative doses (15 mg/kg over 3 weeks) causing more severe damage [6] - A doxorubicin-conjugated anti-HIV-1 antibody reduces viral load in HIV-1-infected humanized mice. Administration of the conjugate (intraperitoneal) leads to a significant decrease in plasma HIV-1 RNA levels and viral replication in lymphoid tissues [7] |
| Enzyme Assay |
Purified human DNA topoisomerase I was assayed quantitatively by enzyme titrations with supercoiled pHC624 DNA in the presence of 0-2.0 microM doxorubicin. Supercoiled and relaxed DNAs were resolved by agarose gel electrophoresis in the presence of ethidium bromide, and the percentage of conversion of supercoiled DNA to relaxed DNA was quantified by scanning microdensitometry. The inhibition of DNA topoisomerase I activity was measured at varying concentrations of doxorubicin. Doxorubicin inhibited enzyme activity at an IC50 value (the concentration required to inhibit 50% of the total activity) of 0.8 microM. Similar inhibition was observed for daunomycin, a structurally related anthracycline antitumor drug. These results indicate that anthracyclines inhibit human DNA topoisomerase I activity at concentrations that cause DNA damage and cytotoxicity in vivo[3].
- Topoisomerase II inhibition assay: Recombinant human topoisomerase II was incubated with supercoiled plasmid DNA and various concentrations of Doxorubicin. The reaction mixture was analyzed by agarose gel electrophoresis to assess DNA relaxation. The formation of cleavable complexes was detected by measuring DNA breakage after protein denaturation [1] - Topoisomerase I inhibition assay: Human topoisomerase I was incubated with supercoiled DNA and Doxorubicin (0.1-100 μM). The extent of DNA relaxation was visualized by agarose gel electrophoresis, and IC50 was calculated based on the concentration required to inhibit 50% of enzyme activity [3] |
| Cell Assay |
Three 96-well U-bottom microplates with a suspension of Hela cells (3×104 cell/mL) dispensed in 160 μL are then incubated for 24 hours at 37°C in a fully humidified atmosphere with 5% CO2. In plate 1, 200 μL of final volume is filled with serial dilutions of doxorubicin (20 μL; final concentration, 0.1-2 μM) and simvastatin (20 μL; final concentration, 0.25-2 μM) before an additional 72 hours of incubation. 40 μL of each drug's serial dilution—doxorubicin or simvastatin—are added to plates 2 and 3. The medium is aspirated and the cells are cleaned in PBS after a 24-hour incubation period. Then, to reach a final volume of 200 μL, serial dilutions of the other medication (40 μL) are added, and the mixture is incubated for 48 hours. Separate positive controls (40 μL in each well) consisting of doxorubicin and simvastatin are employed, while the negative controls consist solely of solvent-treated cells. A 20 μL MTT solution (5 mg/mL in PBS) is added to each well, and the cells are incubated for three hours in order to assess cell survival. Afterwards, 150 μL of DMSO is added to the medium, and the solution is pipetted repeatedly to completely dissolve the formazan crystals. In the next step, an ELISA plate reader measures absorbance at 540 nm. Three assays are conducted, one for each drug concentration, using four or eight wells. Doxorubicin's cytotoxic/cytostatic effect is quantified and expressed as relative viability (% control). It is assumed that 100% of the cells in the negative control will survive. * Relative viability = (background absorbance - experimental absorbance) / (background absorbance - absorbance of untreated controls)× 100%[4].
- Cytotoxicity assay (combination with simvastatin): Human cancer cells (MCF-7, HT-29, HepG2) were seeded in 96-well plates and treated with Doxorubicin (0.01-10 μM) alone, simvastatin (0.1-100 μM) alone, or their combinations. After 72 hours, cell viability was measured using a colorimetric assay, and combination index (CI) was calculated to determine synergism [4] - Apoptosis assay (with Apo2L/TRAIL): Prostate cancer cells (PC-3, DU145) were treated with Doxorubicin (0.1-1 μM) and/or Apo2L/TRAIL (10-100 ng/ml) for 24-48 hours. Apoptosis was assessed by caspase-3 activity assay and annexin V-FITC staining followed by flow cytometry [5] - HIV-1 inhibition assay: HIV-1-infected T cells were treated with doxorubicin-conjugated anti-envelope antibody (0.1-10 μg/ml) for 72 hours. Viral replication was measured by p24 antigen ELISA in culture supernatants [7] |
| Animal Protocol |
Mice: Three to four-week-old male athymic nude mice are used. Subcutaneous injection of PC3 cells (4×106) is administered to mice via the flanks. When the volumes of the xenografts reached approximately 100 mm3, the animals with tumors were randomly assigned to treatment groups, with five or six mice per group. Digital calipers are used to measure tumors and the formula Volume=Width2×Length×0.52 is used to calculate the volume of the tumor, with width denoting its shorter dimensions. Therapy is given as prescribed with vehicle (PBS with 0.1% BSA), Doxorubicin (2–8 mg/kg), Apo2L/TRAIL (500 μg/animal), or a mix of 4 mg/kg Doxorubicin and 500 μg Apo2L/TRAIL. Doxorubicin is delivered systemically, while Apo2L/TRAIL is delivered intra-tumorally or systemically. Every therapy is administered just once. Every day, mice are observed for indications of negative consequences, such as lethargic behavior and disheveled look. Looks like the treatments were well received. Every data point has its mean±SEM computed. Student t-tests are used to analyze differences between treatment groups. When P is less than 0.05, differences are deemed significant.
Rats: Thirty-year-old man A total of ten Doxorubicin schedules—Doxorubicin schedule 1 (n = ten), Doxorubicin schedule 2 (n = ten), and Doxorubicin schedule 3 (n = ten)—are randomly assigned to Sprague-Dawley rats weighing 250–300 g. 10 mg/kg is the total dose of doxorubicin for all treatment regimens. One intraperitoneal injection of doxorubicin (10 mg/kg) is administered as part of Schedule 1. Doxorubicin injections intraperitoneally (10 mg/kg) for ten days in a row are part of Schedule 2. Schedule 2 calls for ten intraperitoneal injections of doxorubicin (1 mg/kg) spaced ten days apart. Schedule 3 calls for five weeks of weekly intraperitoneal injections of doxorubicin at a dose of two milligrams per kilogram. As long as there are at least three rats in each group, blood pressure and cardiac function are measured in all surviving animals prior to the first Doxorubicin treatment and once a week after the start of Doxorubicin treatment.
- Prostate cancer xenograft model: Nude mice were subcutaneously implanted with PC-3 cells. Once tumors reached ~100 mm³, mice were treated with Doxorubicin (2 mg/kg, intraperitoneal, once weekly), Apo2L/TRAIL (15 mg/kg, intravenous, twice weekly), or their combination for 3 weeks. Tumor volume was measured twice weekly, and mice were monitored for survival [5] - Cardiotoxicity model: Rats were administered Doxorubicin (2.5 mg/kg, intraperitoneal) once weekly for 6 weeks (cumulative dose 15 mg/kg) or saline (control). Cardiac function was assessed by echocardiography (left ventricular ejection fraction, fractional shortening) at 2, 4, and 6 weeks. Serum cardiac troponin I levels were measured, and heart tissues were collected for histopathological analysis [6] - HIV-1-infected humanized mice: Mice were infected with HIV-1, then treated with doxorubicin-conjugated anti-envelope antibody (5 mg/kg, intraperitoneal) once weekly for 3 weeks. Plasma and lymphoid tissues were collected to measure viral RNA levels by RT-PCR [7] |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
Following a 10 mg/m2 administration of liposomal doxorubicin in patients with AIDS-related Kaposi's Sarcoma, the Cmax and AUC values were calculated to be 4.12 ± 0.215 μg/mL and 277 ± 32.9 μg/mL•h respectively. Approximately 40% of the dose appears in the bile in 5 days, while only 5% to 12% of the drug and its metabolites appear in the urine during the same time period. In urine, <3% of the dose was recovered as doxorubicinol over 7 days. The steady-state distribution volume of doxorubicin ranges from 809 L/m2 to 1214 L/m2. The plasma clearance of doxorubicin ranges from 324 mL/min/m2 to 809 mL/min/m2 by metabolism and biliary excretion. Sexual differences in doxorubicin were also observed, with men having a higher clearance compared to women (1088 mL/min/m2 versus 433 mL/min/m2). Following the administration of doses ranging from 10 mg/m2 to 75 mg/m2 of doxorubicin hydrochloride, the plasma clearance was estimated to be 1540 mL/min/m2 in children greater than 2 years of age and 813 mL/min/m2 in infants younger than 2 years of age. Nonencapsulated doxorubicin hydrochloride is not stable in gastric acid, and animal studies indicate that the drug undergoes little, if any, absorption from the GI tract. The drug is extremely irritating to tissues and, therefore, must be administered iv. Following iv infusion of a single 10- or 20-mg/sq m dose of liposomal doxorubicin hydrochloride in patients with AIDS-related Kaposi's sarcoma, average peak plasma doxorubicin (mostly bound to liposomes) concentrations are 4.33 or 10.1 ug/mL, respectively, following a 15-minute infusion and 4.12 or 8.34 ug/mL, respectively, following a 30-minute infusion. Following iv infusion over 15 minutes of a 40-mg/sq m dose of liposomal doxorubicin hydrochloride in adults with AIDS-related Kaposi's, peak plasma concentrations averaged 20.1 ug/mL. Nonencapsulated (conventional) doxorubicin hydrochloride exhibits linear pharmacokinetics; PEG-stabilized liposomal doxorubicin hydrochloride also exhibits dose-proportional, linear pharmacokinetics over a dosage range of 10-20 mg/sq m. The pharmacokinetics of liposomally encapsulated doxorubicin at a dose of 50 mg/sq m have been reported to be nonlinear. At a dose of 50 mg/sq m, a longer elimination half-life and lower clearance compared to those observed with a 20 mg/sq m dose are expected, with greater-than-proportional increases in area under the plasma concentration-time curve. Encapsulation of doxorubicin hydrochloride in PEG-stabilized (Stealth) liposomes substantially alters the pharmacokinetics of the drug relative to conventional iv formulations (ie, nonencapsulated drug), with resultant decreased distribution into the peripheral compartment, increased distribution into Kaposi's lesions, and decreased plasma clearance. Doxorubicin administered as a conventional injection is widely distributed in the plasma and in tissues. As early as 30 seconds after iv administration, doxorubicin is present in the liver, lungs, heart, and kidneys. Doxorubicin is absorbed by cells and binds to cellular components, particularly to nucleic acids. The volume of distribution of doxorubicin hydrochloride administered iv as a conventional injection is about 700-1100 L/sq m. Nonencapsulated doxorubicin is approximately 50-85% bound to plasma proteins... Doxorubicin hydrochloride administered iv as the liposomally encapsulated drug distributes into Kaposi's sarcoma lesions to a greater extent than into healthy skin. Following iv administration of a single 20-mg/sq m dose of liposomal doxorubicin hydrochloride, doxorubicin concentrations in Kaposi's sarcoma lesions were 19 (range: 3-53)-fold higher than those observed in healthy skin; however, blood concentrations in the lesions or in healthy skin were not considered. In addition, distribution of doxorubicin into Kaposi's sarcoma lesions following iv administration of liposomally encapsulated drug was 5.2-11.4 times greater than that following iv administration of comparable doses of a conventional (nonencapsulated) injection. The mechanism by which liposomal encapsulation enhances doxorubicin distribution into Kaposi's sarcoma lesions has not been elucidated fully, but similar PEG-stabilized liposomes containing colloidal gold as a marker have been shown to enter Kaposi's sarcoma-like lesions in animals. Extravasation of the liposomes also may occur by passage of the particles through endothelial cell gaps present in Kaposi's sarcoma. Once within the lesions, the drug presumably is released locally as the liposomes degrade and become permeable in situ. For more Absorption, Distribution and Excretion (Complete) data for DOXORUBICIN (16 total), please visit the HSDB record page. Metabolism / Metabolites Doxorubicin is capable of undergoing 3 metabolic routes: one-electron reduction, two-electron reduction, and deglycosidation. However, approximately half of the dose is eliminated from the body unchanged. The two-electron reduction is the major metabolic pathway of doxorubicin. In this pathway, doxorubicin is reduced to doxorubicinol, a secondary alcohol, by various enzymes, including Alcohol dehydrogenase [NADP(+)], Carbonyl reductase [NADPH] 1, Carbonyl reductase [NADPH] 3, and Aldo-keto reductase family 1 member C3. The one-electron reduction is facilitated by several oxidoreductase, both cytosolic and mitochondrial, to form a doxirubicin-semiquinone radical. These enzymes include mitochondrial and cystolic NADPH dehydrogenates, xanthine oxidase, and nitric oxide synthases. This semiquinone metabolite can be re-oxidized to doxorubicin, although with the concurrent formation of reactive oxygen species (ROS) and hydrogen peroxide. It is the ROS generating through this pathway that contributes most to the doxorubicin-related adverse effects, particularly cardiotoxicity, rather than through doxorubicin semiquinone formation. Deglycosidation is a minor metabolic pathway, since it only accounts for 1 to 2% of doxorubicin metabolism. Under the catalysis of cytoplasmic NADPH quinone dehydrogenase, xanthine oxidase, NADPH-cytochrome P450 reductase, doxorubicin can either be reduced to doxorubicin deoxyaglycone or hydrolyzed to doxorubicin hydroxyaglycone. Nonencapsulated doxorubicin is metabolized by NADPH-dependent aldoketoreductases to the hydrophilic 13-hydroxyl metabolite doxorubicinol, which exhibits antineoplastic activity and is the major metabolite; these reductases are present in most if not all cells, but particularly in erythrocytes, liver, and kidney. Although not clearly established, doxorubicinol also appears to be the moiety responsible for the cardiotoxic effects of the drug. Undetectable or low plasma concentrations (ie, 0.8-26.2 ng/mL) of doxorubicinol have been reported following iv administration of a single 10- to 50-mg/sq m dose of doxorubicin hydrochloride as a PEG-stabilized liposomal injection; it remains to be established whether such liposomally encapsulated anthracyclines are less cardiotoxic than conventional (nonencapsulated) drug, and the usual precautions for unencapsulated drug currently also should be observed for the liposomal preparation. Substantially reduced or absent plasma concentrations of the usual major metabolite of doxorubicin observed with the PEG-stabilized liposomal injection suggests that either the drug is not released appreciably from the liposomes as they circulate or that some doxorubicin may be released but that the rate of doxorubicinol elimination greatly exceeds the release rate; doxorubicin hydrochloride encapsulated in liposomes that have not been PEG-stabilized is metabolized to doxorubicinol. Other metabolites, which are therapeutically inactive, include the poorly water-soluble aglycones, doxorubicinone (adriamycinone) and 7-deoxydoxorubicinone (17-deoxyadriamycinone), and conjugates. The aglycones are formed in microsomes by NADPH-dependent, cytochrome reductase-mediated cleavage of the amino sugar moiety. The enzymatic reduction of doxorubicin to 7-deoxyaglycones is important to the cytotoxic effect of the drug since it results in hydroxyl radicals that cause extensive cell damage and death. With nonencapsulated doxorubicin, more than 20% of the total drug in plasma is present as metabolites as soon as 5 minutes after a dose, 70% in 30 minutes, 75% in 4 hours, and 90% in 24 hours. ... At least 6 metabolites have been identified, the principal one being adriamycinol. This product results from redn of the keto group on C13 by an enzyme found in leukocytes and erythrocytes, and presumably in malignant tissues. Doxorubicin is converted to doxorubicinol, to aglycones, and to other derivatives For more Metabolism/Metabolites (Complete) data for DOXORUBICIN (6 total), please visit the HSDB record page. Doxorubicin is capable of undergoing 3 metabolic routes: one-electron reduction, two-electron reduction, and deglycosidation. However, approximately half of the dose is eliminated from the body unchanged. Two electron reduction yields doxorubicinol, a secondary alcohol. This pathway is considered the primary metabolic pathway. The one electron reduction is facilitated by several oxidoreductases to form a doxirubicin-semiquinone radical. These enzymes include mitochondrial and cystolic NADPH dehydrogenates, xanthine oxidase, and nitric oxide synthases. Deglycosidation is a minor metabolic pathway (1-2% of the dose undergoes this pathway). The resultant metabolites are deoxyaglycone or hydroxyaglycone formed via reduction or hydrolysis respectively. Enzymes that may be involved with this pathway include xanthine oxidase, NADPH-cytochrome P450 reductase, and cytosolic NADPH dehydrogenase. Route of Elimination: 40% of the dose appears in bile in 5 days. 5-12% of the drug and its metabolites appears in urine during the same time period. <3% of the dose recovered in urine was doxorubicinol. Half Life: Terminal half life = 20 - 48 hours. Biological Half-Life The terminal half-life of doxorubicin ranges from 20 hours to 48 hours. The distribution half-life of doxorubicin is approximately 5 minutes. For the liposomal formulation, the first-phase and second-phase half-lives were calculated to be 4.7 ± 1.1 and 52.3 ± 5.6 hours respectively for a 10 mg/m2 of doxorubicin in patients with AIDS-Related Kaposi’s Sarcoma. Plasma concentrations of nonencapsulated doxorubicin and its metabolites decline in a biphasic or triphasic manner. In the first phase of the triphasic model, nonencapsulated doxorubicin is rapidly metabolized, presumably by a first-pass effect through the liver. It appears that most of this metabolism is completed before the entire dose is administered. In the triphasic model, nonencapsulated doxorubicin and its metabolites are rapidly distributed into the extravascular compartment with a plasma half-life of approximately 0.2-0.6 hours for doxorubicin and 3.3 hours for its metabolites. This is followed by relatively prolonged plasma concentrations of doxorubicin and its metabolites, probably resulting from tissue binding. During the second phase, the plasma half-life of nonencapsulated doxorubicin is 16.7 hours and that of its metabolites is 31.7 hours. In the biphasic model, the initial distribution t1/2 has been reported to average about 5-10 minutes, and the terminal elimination t1/2 has been reported to average about 30 hours. Plasma concentrations of liposomally encapsulated doxorubicin hydrochloride appear to decline in a biphasic manner. Following iv administration of a single 10- to 40-mg/sq m dose of doxorubicin hydrochloride as a liposomal injection in patients with AIDS-related Kaposi's sarcoma, the initial plasma half-life (t1/2 alpha) of doxorubicin averaged 3.76-5.2 hours while the terminal elimination half-life (t1/2 beta) averaged 39.1-55 hours. The initial distribution half-life of approximately 5 minutes suggests rapid tissue uptake of doxorubicin, while its slow elimination from tissues is reflected by a terminal half-life of 20 to 48 hours. Plasma T/2 of Adriamycin is about 17 hr in patient, whereas that of its metabolites is about 32 hr. |
| Toxicity/Toxicokinetics |
Toxicity Summary
Doxorubicin has antimitotic and cytotoxic activity through a number of proposed mechanisms of action: Doxorubicin forms complexes with DNA by intercalation between base pairs, and it inhibits topoisomerase II activity by stabilizing the DNA-topoisomerase II complex, preventing the religation portion of the ligation-religation reaction that topoisomerase II catalyzes. Toxicity Data LD50: 21 800 ug/kg (Subcutaneous, Rat) (A308) Interactions There have been a number of reports in the literature that describe an increase in cardiotoxicity when doxorubicin is co-administered with paclitaxel. Two published studies report that initial administration of paclitaxel infused over 24 hours followed by doxorubicin administered over 48 hours resulted in a significant decrease in doxorubicin clearance with more profound neutropenic and stomatitis episodes than the reverse sequence of administration. In a published study, progesterone was given intravenously to patients with advanced malignancies (ECOG PS<2) at high doses (up to 10 g over 24 hours) concomitantly with a fixed doxorubicin dose (60 mg/sq m) via bolus injection. Enhanced doxorubicin-induced neutropenia and thrombocytopenia were observed. A study of the effects of verapamil on the acute toxicity of doxorubicin in mice revealed higher initial peak concentrations of doxorubicin in the heart with a higher incidence and severity of degenerative changes in cardiac tissue resulting in a shorter survival. The addition of cyclosporine to doxorubicin may result in increases in AUC for both doxorubicin and doxorubicinol possibly due to a decrease in clearance of parent drug and a decrease in metabolism of doxorubicinol. Literature reports suggest that adding cyclosporine to doxorubicin results in more profound and prolonged hematologic toxicity than doxorubicin alone. Coma and/or seizures have also been described. For more Interactions (Complete) data for DOXORUBICIN (16 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat ip 16 mg/kg LD50 Rat iv 12.6 mg/kg LD50 Mouse oral 570 mg/kg LD50 Mouse ip 10,700 ug/kg For more Non-Human Toxicity Values (Complete) data for DOXORUBICIN (8 total), please visit the HSDB record page. - Doxorubicin induces cardiotoxicity in rats, characterized by reduced left ventricular function, increased cardiac troponin I, and myocardial histopathological changes (vacuolization, fibrosis) at cumulative doses of 15 mg/kg [6] - Plasma protein binding of Doxorubicin is approximately 75-85% [1] |
| References | |
| Additional Infomation |
Therapeutic Uses
Antibiotics; Antineoplastic Agents Doxorubicin has been used successfully to produce regression in disseminated neoplastic conditions such as acute lymphoblastic leukemia, acute myeloblastic leukemia, Wilms' tumor, neuroblastoma, soft tissue and bone sarcomas, breast carcinoma, ovarian carcinoma, transitional cell bladder carcinoma, thyroid carcinoma, gastric carcinoma, Hodgkin's disease, malignant lymphoma and bronchogenic carcinoma in which the small cell histologic type is the most responsive compared to other cell types. /Included in US Product label/ Doxorubicin is also indicated for use as a component of adjuvant therapy in women with evidence of axillary lymph node involvement following resection of primary breast cancer. /Included in US product label/ DOXIL (doxorubicin hydrochloride, liposomal) is an anthracycline topoisomerase inhibitor indicated for ovarian cancer after failure of platinum-based chemotherapy. /Included in US product label/ For more Therapeutic Uses (Complete) data for DOXORUBICIN (7 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ WARNING: CARDIOMYOPATHY. Myocardial damage, including acute left ventricular failure can occur with doxorubicin hydrochloride. The risk of cardiomyopathy is proportional to the cumulative exposure with incidence rates from 1% to 20% for cumulative doses ranging from 300 mg/sq m to 500 mg/sq m when doxorubicin hydrochloride is administered every 3 weeks. The risk of cardiomyopathy is further increased with concomitant cardiotoxic therapy. Assess LVEF before and regularly during and after treatment with doxorubicin hydrochloride. /BOXED WARNING/ WARNING: SECONDARY MALIGNANCIES. Secondary acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS) occur at a higher incidence in patients treated with anthracyclines, including doxorubicin hydrochloride /BOXED WARNING/ WARNING: EXTRAVASATION AND TISSUE NECROSIS. Extravasation of doxorubicin hydrochloride can result in severe local tissue injury and necrosis requiring wide excision of the affected area and skin grafting. Immediately terminate the drug and apply ice to the affected area. /BOXED WARNING/ WARNING: SEVERE MYELOSUPPRESSION. Severe myelosuppression resulting in serious infection, septic shock, requirement for transfusions, hospitalization, and death may occur. For more Drug Warnings (Complete) data for DOXORUBICIN (65 total), please visit the HSDB record page. Pharmacodynamics Doxorubicin is a cytotoxic, cell-cycle non-specific anthracycline antibiotic. It is generally thought to exert its antitumor effect by destabilizing DNA structures through intercalation, thus introducing DNA strand breakages and damages. Not only does it alter the transcriptomes of the cells, failure in repairing DNA structures can also initiate the apoptotic pathways. Additionally, doxorubicin intercalation can also interfere with vital enzyme activity, such as topoisomerase II, DNA polymerase, and RNA polymerase, leading to cell cycle arrests. Finally, doxorubicin can also generate cytotoxic reactive oxygen species to exert cellular damages. - Doxorubicin is an anthracycline chemotherapy agent widely used in cancer treatment. Its primary mechanism involves inhibition of DNA topoisomerase II, leading to DNA damage and cell death. It is effective against various solid tumors and hematological malignancies but is limited by cardiotoxicity [1] - Doxorubicin can be conjugated to antibodies (e.g., anti-HIV-1 envelope antibody) for targeted delivery, enhancing efficacy against specific cells (e.g., HIV-1-infected cells) while reducing off-target toxicity [7] |
| Molecular Formula |
C27H29NO11
|
|
|---|---|---|
| Molecular Weight |
543.52
|
|
| Exact Mass |
543.17
|
|
| Elemental Analysis |
C, 59.66; H, 5.38; N, 2.58; O, 32.38.
|
|
| CAS # |
23214-92-8
|
|
| Related CAS # |
|
|
| PubChem CID |
31703
|
|
| Appearance |
Deep-red to black solid powder
|
|
| Density |
1.61 g/cm3
|
|
| Melting Point |
205ºC
|
|
| Flash Point |
443.8ºC
|
|
| Vapour Pressure |
9.64E-28mmHg at 25°C
|
|
| Index of Refraction |
1.709
|
|
| LogP |
1.503
|
|
| Hydrogen Bond Donor Count |
6
|
|
| Hydrogen Bond Acceptor Count |
12
|
|
| Rotatable Bond Count |
5
|
|
| Heavy Atom Count |
39
|
|
| Complexity |
977
|
|
| Defined Atom Stereocenter Count |
6
|
|
| SMILES |
[H][C@@]1(O[C@H]2C[C@](O)(C(CO)=O)CC(C2=C3O)=C(O)C4=C3C(C5=C(OC)C=CC=C5C4=O)=O)O[C@@H](C)[C@@H](O)[C@@H](N)C1
|
|
| InChi Key |
AOJJSUZBOXZQNB-TZSSRYMLSA-N
|
|
| InChi Code |
InChI=1S/C27H29NO11/c1-10-22(31)13(28)6-17(38-10)39-15-8-27(36,16(30)9-29)7-12-19(15)26(35)21-20(24(12)33)23(32)11-4-3-5-14(37-2)18(11)25(21)34/h3-5,10,13,15,17,22,29,31,33,35-36H,6-9,28H2,1-2H3/t10-,13-,15-,17-,22+,27-/m0/s1
|
|
| Chemical Name |
(7S,9S)-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione
|
|
| Synonyms |
Adriamycin; Hydroxydaunorubicin; ADR; DOX. Code name: FI106; chloridrato de doxorrubicina. Adriamycin; Adriacin; Adriblastina; Adriblastine; Adrimedac; DOXOCELL; Doxolem; Doxorubin; Farmiblastina; Rubex. Abbreviations: ADM; Adria;
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples.
Injection Formulations
Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline)(e.g. IP/IV/IM/SC) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). View More
Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] Oral Formulations
Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). View More
Oral Formulation 3: Dissolved in PEG400 (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8399 mL | 9.1993 mL | 18.3986 mL | |
| 5 mM | 0.3680 mL | 1.8399 mL | 3.6797 mL | |
| 10 mM | 0.1840 mL | 0.9199 mL | 1.8399 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
Interest of Post-operative Chemotherapy in Patients With Localised Uterine Leiomyosarcoma Suspected of Having a High Risk of Recurrence Based on a Biological Test Performed on the Tumour
CTID: NCT06524583
Phase: Phase 2 Status: Not yet recruiting
Date: 2024-12-02
 |
|---|
|