
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
Purity: ≥98%
Sotorasib racemate (AMG-510; AMG510), the racemic mixture of AMG-510, is a novel, potent and covalent inhibitor of KRAS G12C. The FDA approved Sotorasib's active isomer on May 28, 2021, for the treatment of non-small-cell lung cancer (NSCLC). Mutations in the KRAS gene are frequently found in a variety of cancers, including colorectal, thyroid, pancreatic, lung adenocarcinoma, gall bladder, and bile duct cancers.
| Targets |
KRas G12C; Mutant KRAS G12C (irreversibly binds to the switch II pocket, Ki = 11 nM for KRAS G12C-GDP; IC50 = 0.21 μM for inhibiting KRAS G12C-mediated signaling in H358 cells) [6]
|
|---|---|
| ln Vitro |
AMG 510 had little effect on KRAS (C118A) but inhibits the nucleotide exchange of recombinant mutant KRAS (G12C/C118A) when catalyzed by SOS1. Additionally, AMG 510 specifically reduces the viability of KRAS p.G12C mutant lines while having no effect on cell lines harboring other KRAS mutations[1].
- Sotorasib (AMG-510) potently inhibited proliferation of KRAS G12C-positive cancer cell lines, with IC50 values of 0.01-0.5 μM (e.g., H358 lung adenocarcinoma: 0.03 μM; MIA PaCa-2 pancreatic cancer: 0.12 μM) as measured by CellTiter-Glo assay. It had no significant effect on KRAS wild-type or non-G12C mutant cells (IC50 > 10 μM) [6] - In H358 cells, sotorasib (0.1-1 μM) dose-dependently reduced phosphorylation of KRAS downstream effectors (p-ERK, p-AKT, p-S6) within 2 hours (Western blot), with maximal inhibition at 1 μM [6] - The compound (1 μM) induced apoptosis in KRAS G12C-positive cells (Annexin V/PI staining) and reduced colony formation (by 80% in H358 cells) compared to vehicle [6] |
| ln Vivo |
AMG 510 quickly and irreversibly binds to KRAS (G12C) in preclinical tumor models, resulting in long-lasting inhibition of the mitogen-activated protein kinase (MAPK) signaling pathway. When administered orally (once daily) as a single agent, AMG 510 has the ability to induce tumor regression in KRASG12C cancer-bearing mice models[2].
- In mice bearing H358 xenografts (KRAS G12C), sotorasib (10-180 mg/kg, oral gavage, daily for 21 days) caused dose-dependent tumor regression: 180 mg/kg led to 90% tumor growth inhibition (TGI) and 30% complete regression. No significant effect was observed in KRAS wild-type A549 xenografts [6] - In a patient-derived xenograft (PDX) model of KRAS G12C colorectal cancer, sotorasib (100 mg/kg, oral) reduced tumor volume by 65% after 28 days, with decreased p-ERK levels in tumor tissues (immunohistochemistry) [3] |
| Enzyme Assay |
Activating mutations in RAS represent the most common oncogenic driver mutation in cancer. The single amino acid substitution of cysteine for glycine at position 12 (KRASG12C) is frequently found in solid malignancies, particularly in lung adenocarcinoma (~13%), colorectal adenocarcinoma (3%), and pancreatic adenocarcinoma (~1%). Recently it has been demonstrated that KRASG12C can be targeted with covalent small molecule inhibitors which react with the mutant cysteine adjacent to the switch II pocket (SIIP), locking KRAS in its inactive GDP-bound state. We describe here the discovery and in vitro characterization of AMG 510, a covalent inhibitor of KRASG12C possessing potent biochemical and cellular activity, as well as robust in vivo efficacy. AMG 510 inhibited SOS1-catalyzed nucleotide exchange of recombinant mutant KRASG12C/C118A but had minimal effect on KRASC118A, which is wildtype at position 12. The observed rate constant (kinact/Ki) of covalent modification of KRASG12C by AMG 510 was determined biochemically by mass spectrometry as well as in the cellular context (kobs/[I]). Cysteine proteome analysis of cells treated with AMG 510 revealed that only the G12C-containing peptide of KRAS was covalently modified. AMG 510 inhibited KRAS signaling as measured by ERK phosphorylation in all KRAS p.G12C cell lines tested, but did not inhibit phosphorylation of ERK in cell lines lacking the KRAS p.G12C mutation. Cellular occupancy of KRASG12C by AMG 510 was determined by mass spectrometry and correlated well with inhibition of ERK phosphorylation. AMG 510 also selectively impaired the viability of KRAS p.G12C mutant lines. Combination treatment of AMG 510 with inhibitors of other cellular signaling pathways exhibited evidence for synergistic effects on cell viability. Treatment of KRAS p.G12C lines with covalent KRASG12C inhibitors increased the expression of HLA. To test the impact of KRASG12C inhibition on immune surveillance in vivo, we generated a syngeneic tumor cell line that is suitable for testing AMG 510 in combination with checkpoint inhibitor therapies and characterized this line in vitro. AMG 510 is currently being evaluated in a Phase I study in patients with solid tumors harboring KRAS p.G12Cmutations[1].
KRAS G12C binding assay: Purified KRAS G12C-GDP protein was incubated with sotorasib (0.1-100 nM) and analyzed by surface plasmon resonance (SPR). The compound showed slow dissociation (t1/2 = 10 hours) with a Ki of 11 nM. GTPase activity was measured using a luminescent GTP hydrolysis assay, where sotorasib (1 μM) inhibited KRAS G12C GTPase activity by 85% [6] |
| Cell Assay |
- Proliferation assay: KRAS G12C-positive cells (H358, MIA PaCa-2) were seeded in 96-well plates and treated with sotorasib (0.001-10 μM) for 72 hours. Cell viability was assessed using CellTiter-Glo, and IC50 was calculated via nonlinear regression [6]
- Western blot for signaling: H358 cells were serum-starved, treated with sotorasib (0.1-1 μM) for 2 hours, then lysed. Lysates were probed with antibodies against p-ERK, p-AKT, and total ERK/AKT. Band intensities were normalized to β-actin [6] |
| Animal Protocol |
- Xenograft model: Nude mice were subcutaneously injected with H358 cells (5×10⁶). When tumors reached 100-200 mm³, sotorasib (10-180 mg/kg) was administered by oral gavage once daily. Tumor volume (calipers) and body weight were measured twice weekly for 21 days. Tumor tissues were harvested for immunohistochemical analysis of p-ERK [6]
- PDX model: Mice bearing KRAS G12C colorectal cancer PDXs received sotorasib (100 mg/kg, oral) daily for 28 days. Tumor growth was monitored, and Ki-67 (proliferation marker) expression was quantified [3] The RAS gene family encodes the small GTPase proteins NRAS, HRAS, and KRAS, which play an essential role in cellular growth and proliferation. KRAS is one of the most frequently mutated oncogenes in human cancer, with KRAS p.G12D, p.G12V, and p.G12C constituting the major mutational subtypes across lung, colon, and pancreatic cancers. Despite more than three decades of research, indirect approaches targeting KRAS mutant cancers have largely failed to show clinical benefit, and direct approaches have been stymied by the apparently ‘undruggable’ nature of KRAS. Cysteine-12 of KRASG12C has recently emerged as a unique vulnerability in KRAS-mutant cancers, and a small number of cysteine-reactive inhibitory tool molecules have been disclosed. We here report independent efforts to identify cysteine-reactive molecules capable of selectively inhibiting KRASG12C. Through iterative screening and structural biology efforts, we identified a novel Cys12-reactive inhibitor scaffold that derived its potency from occupancy of a previously unknown cryptic pocket induced by side-chain motion of the His95 residue of KRAS. Employing a scaffold-hopping approach, we leveraged knowledge of this cryptic pocket to design a series of N-aryl quinazolin-2(1H)-one-based inhibitors that demonstrated significantly enhanced potency relative to prior tool compounds. Extensive optimization of these leads led to the identification of a highly potent, selective, and well-tolerated inhibitor of KRASG12C, which was nominated for clinical development as AMG 510. In preclinical tumor models, AMG 510 rapidly and irreversibly binds to KRASG12C, providing durable suppression of the mitogen-activated protein kinase (MAPK) signaling pathway. Dosed orally (once daily) as a single agent, AMG 510 is capable of inducing tumor regression in mouse models of KRASG12C cancer. AMG 510 is, to the best of our knowledge, the first direct KRASG12C therapeutic to reach human clinical testing and is currently in a Phase I clinical trial evaluating safety, tolerability, PK, and efficacy in subjects with solid tumors bearing the KRAS p.G12C mutation (NCT03600883)[2]. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
A 960 mg once daily dose of sotorasib reaches a Cmax of 7.50 µg/mL, with a median Tmax of 2.0 hours, and an AUC0-24h of 65.3 h\*µg/mL. Sotorasib is 74% eliminated in the feces and 6% eliminated in the urine. 53% of the dose recovered in the feces and 1% of the dose recovered in the urine is in the form of the unchanged parent compound. The volume of distribution of sotorasib is 211 L. Sotorasib has an apparent clearance at steady state of 26.2 L/h. Metabolism / Metabolites Sotorasib is predominantly metabolized through conjugation or by CYP3As. Biological Half-Life Sotorasib has a terminal elimination half life of 5.5 ± 1.8 hours. - In mice, oral administration of sotorasib (10 mg/kg) showed 70% bioavailability, with peak plasma concentration (Cmax) of 2.3 μg/mL at 1 hour. It had a plasma half-life (t1/2) of 4.5 hours and good tumor penetration (tumor/plasma ratio = 3.2) [6] - In patients, sotorasib (960 mg, oral) reached Cmax of 7.1 μg/mL at 1.5 hours, with a terminal t1/2 of 5 hours. Plasma protein binding was 95% [4] |
| Toxicity/Toxicokinetics |
Hepatotoxicity
In the prelicensure clinical trials of sotorasib in patients with solid tumors harboring KRAS G12C mutations, liver test abnormalities were frequent although usually self-limited and mild. Some degree of ALT elevations arose in 38% of sotorasib treated patients and were above 5 times the upper limit of normal (ULN) in 6% to 7%. In these trials that enrolled approximately 427 patients, sotorasib was discontinued early due to increased AST or ALT in 8% of patients. In addition, a small proportion of patients developed significant hepatotoxicity requiring sotorasib discontinuation and treatment with corticosteroids. The liver test abnormalities had a median onset of 9 weeks after initiation of therapy. While serum aminotransferase elevations were occasionally quite high (5 to 20 times upper limit of normal), there was no accompanying elevations in serum bilirubin and no patient developed clinically apparent liver injury with jaundice. The product label for sotorasib recommends monitoring for routine liver tests before, at 3 week intervals during the first 3 months of therapy, and monthly thereafter as clinically indicated. Strikingly, the more severe elevations of serum aminotransferase levels during therapy with sotorasib occurred among patients who had recently received checkpoint inhibitor therapy (usually anti-PD-L1) in the 1 to 3 months before starting sotorasib. Furthermore, the elevations tended to respond quickly to corticosteroid therapy and sometimes did not recur when sotorasib was restarted several months later. These findings suggest that the aminotransferase elevations during sotorasib therapy are due to a delayed immune-mediated hepatotoxicity triggered by the previous checkpoint inhibitor therapy. Likelihood score: D (possible but infrequent cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of sotorasib during breastfeeding. Because sotorasib is 89% bound to plasma proteins, the amount in milk is likely to be low. However, because of its potential toxicity in the breastfed infant, the manufacturer recommends that breastfeeding be discontinued during sotorasib therapy and for 1 week after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Sotorasib is 89% protein bound in plasma. - In preclinical studies, sotorasib (up to 300 mg/kg, oral) showed no significant toxicity in mice, with normal liver/kidney function markers [6] - In clinical trials, common adverse events (≥15%) included diarrhea (34%), nausea (25%), and fatigue (21%). Grade 3/4 toxicities were rare (<5%), with no dose-limiting nephrotoxicity or hepatotoxicity [4] |
| References | |
| Additional Infomation |
Sotorasib is a pyridopyrimidine that is pyrido[2,3-d]pyrimidin-2(1H)-one substituted by 4-methyl-2-(propan-2-yl)pyridin-3-yl, (2S)-2-methyl-4-(prop-2-enoyl)piperazin-1-yl, fluoro and 2-fluoro-6-hydroxyphenyl groups at positions 1, 4, 6 and 7, respectively. It is approved for the treatment of patients with non-small cell lung cancer having KRAS(G12C) mutations. It has a role as an antineoplastic agent. It is a member of acrylamides, a N-acylpiperazine, a pyridopyrimidine, a member of monofluorobenzenes, a member of methylpyridines, a tertiary carboxamide, a tertiary amino compound and a member of phenols.
Sotorasib, also known as AMG-510, is an acrylamide-derived KRAS inhibitor developed by Amgen. It is indicated in the treatment of adult patients with KRAS G12C mutant non-small cell lung cancer. This mutation makes up >50% of all KRAS mutations. Mutant KRAS discovered in 1982 but was not considered a druggable target until the mid-2010s. It is the first experimental KRAS inhibitor. The drug [MRTX849] is also currently being developed and has the same target. Sotorasib was granted FDA approval on May 28, 2021, followed by the European Commission's approval on January 10, 2022. Sotorasib is a small molecule inhibitor of the KRAS G12C mutant protein which is found in up to 13% of refractory cases of non-small cell lung cancer. Serum aminotransferase elevations are common during therapy with sotorasib, and a proportion of patients develop clinically apparent liver injury that can be severe. Sotorasib is an orally available inhibitor of the specific KRAS mutation, p.G12C, with potential antineoplastic activity. Upon oral administration, sotorasib selectively targets, binds to and inhibits the activity of the KRAS p.G12C mutant. This may inhibit growth in KRAS p.G12C-expressing tumor cells. The KRAS p.G12C mutation is seen in some tumor cell types and plays a key role in tumor cell proliferation. Drug Indication Sotorasib is indicated in the treatment of KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC) in adults who have received at least one prior systemic therapy. Lumykras as monotherapy is indicated for the treatment of adults with advanced non-small cell lung cancer (NSCLC) with KRAS G12C mutation and who have progressed after at least one prior line of systemic therapy. Mechanism of Action Normally GTP binds to KRAS, activating the protein and promoting effectors to the MAP kinase pathway. GTP is hydrolyzed to GDP, and KRAS is inactivated. KRAS G12C mutations impair hydrolysis of GTP, leaving it in the active form. Sotorasib binds to the cysteine residue in KRAS G12C mutations, holding the protein in its inactive form. The cysteine residue that sotorasib targets is not present in the wild type KRAS, which prevents off-target effects. This mutation is present in 13% of non small cell lung cancer, 3% of colorectal and appendix cancer, and 1-3% of solid tumors. - Sotorasib (AMG-510) is a first-in-class covalent inhibitor that irreversibly binds to the GDP-bound form of KRAS G12C, locking it in an inactive state [6] - It is indicated for treating KRAS G12C-mutated non-small cell lung cancer (NSCLC) after prior systemic therapy, with FDA approval in 2021 [3][4] |
| Molecular Formula |
C30H30F2N6O3
|
|---|---|
| Molecular Weight |
560.594413280487
|
| Exact Mass |
560.23
|
| Elemental Analysis |
C, 64.28; H, 5.39; F, 6.78; N, 14.99; O, 8.56
|
| CAS # |
2252403-56-6
|
| Related CAS # |
Sotorasib;2296729-00-3;Sotorasib isomer
|
| PubChem CID |
137278711
|
| Appearance |
Light yellow to yellow solid powder
|
| LogP |
4
|
| Hydrogen Bond Donor Count |
1
|
| Hydrogen Bond Acceptor Count |
7
|
| Rotatable Bond Count |
5
|
| Heavy Atom Count |
41
|
| Complexity |
1030
|
| Defined Atom Stereocenter Count |
1
|
| SMILES |
C[C@H]1CN(CCN1C2=NC(=O)N(C3=NC(=C(C=C32)F)C4=C(C=CC=C4F)O)C5=C(C=CN=C5C(C)C)C)C(=O)C=C
|
| InChi Key |
NXQKSXLFSAEQCZ-SFHVURJKSA-N
|
| InChi Code |
InChI=1S/C30H30F2N6O3/c1-6-23(40)36-12-13-37(18(5)15-36)28-19-14-21(32)26(24-20(31)8-7-9-22(24)39)34-29(19)38(30(41)35-28)27-17(4)10-11-33-25(27)16(2)3/h6-11,14,16,18,39H,1,12-13,15H2,2-5H3/t18-/m0/s1
|
| Chemical Name |
6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-propan-2-ylpyridin-3-yl)-4-[(2S)-2-methyl-4-prop-2-enoylpiperazin-1-yl]pyrido[2,3-d]pyrimidin-2-one
|
| Synonyms |
Sotorasib; AMG510; AMG-510 racemate; AMG 510
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment (e.g. under nitrogen), avoid exposure to moisture. |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
DMSO: 3.9~100 mg/mL (6.9~178.4 mM)
Ethanol: ~100 mg/mL (~178.4 mM) |
|---|---|
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples.
Injection Formulations
Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline)(e.g. IP/IV/IM/SC) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). View More
Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] Oral Formulations
Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). View More
Oral Formulation 3: Dissolved in PEG400 (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7838 mL | 8.9192 mL | 17.8383 mL | |
| 5 mM | 0.3568 mL | 1.7838 mL | 3.5677 mL | |
| 10 mM | 0.1784 mL | 0.8919 mL | 1.7838 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
|
|---|
AMG 510 inhibits ERK phosphorylation and growth of KRASG12C-mutant tumours in vivo.Nature. 2019 Nov;575(7781):217-223. |
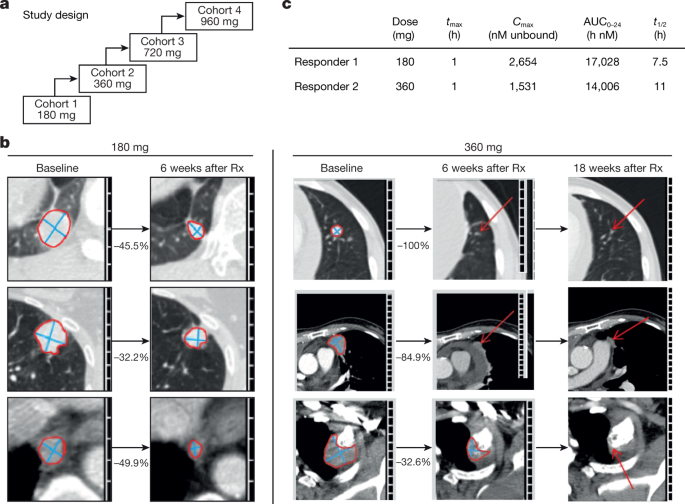 Clinical activity of AMG 510 in patients with lung cancer in first-in-human dose-escalation study.Nature. 2019 Nov;575(7781):217-223. Clinical activity of AMG 510 in patients with lung cancer in first-in-human dose-escalation study.Nature. 2019 Nov;575(7781):217-223. |