| Size | Price | Stock | Qty |
|---|---|---|---|
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
Purity: ≥98%
α-Lipoic Acid (Thioctic acid; (±)-α-Lipoic acid; DL-α-Lipoic acid) is a novel organosulfur compound which acts as an antioxidant, and is an essential cofactor of mitochondrial enzyme complexes. α-Lipoic Acid prevents NF-κB-dependent HIV-1 LTR activation.
| Targets |
Human Endogenous Metabolite; NF-κB; Mitochondrial bioenergetics; HIV-1
|
|---|---|
| ln Vitro |
The long terminal repeat (LTR) of HIV-1 is the target of cellular transcription factors such as NF-κB, and serves as the promoter-enhancer for the viral genome when integrated in host DNA[1]. The dithiol compound α-Lipoic Acid (Alpha-lipoic acid, ALA), which is a naturally occurring substance, is crucial to mitochondrial bioenergetics. By controlling the transcriptional factors SREBP-1, FoxO1, and Nrf2 and their downstream lipogenic targets through the activation of the SIRT1/LKB1/AMPK pathway, α-Lipoic Acid reduces lipid accumulation in the liver. The NAD+/NADH ratio in HepG2 cells is markedly elevated following treatment with -lipoic acid (250, 500, and 1000 μM) (P<0.05 or P<0.01). In HepG2 cells, treatment with -lipoic acid (50, 125, 250, and 500 μM) increases SIRT1 activity. In HepG2 cells, α-Lipoic Acid (50, 125, 250, 500, and 1000 μM) increases AMPK and acetyl-CoA carboxylase (ACC) phosphorylation in a dose-dependent manner[1].
Hepatocellular carcinoma (HCC) is the most common liver cancer and a major cause of adult death. The current treatments for HCC suffer from drug resistance and poor prognosis; therefore, novel therapeutic agents are urgently needed. Phytochemicals have been proposed to treat a range of cancers. Among them, α-lipoic acid (α-LA), a naturally synthesized antioxidant found in various dietary animal and plant sources, prevents oxidant-mediated cell death in normal cells while inducing apoptosis in several cancer cell lines. Previously, we demonstrated that the treatment of hepatoma cells with α-LA induced apoptosis, which was preceded by the generation of reactive oxygen species (ROS) and activation of the p53 protein, a known inducer of mitochondria-mediated apoptosis. Several studies have shown that ROS-induced apoptosis is associated with endoplasmic reticulum (ER) stress and Unfolded Protein Response (UPR) activation. Herein, we investigated if α-LA-induced apoptosis in hepatoma cell lines was ER stress- and UPR-mediated by gene expression profiling analyses. UPR and ER stress pathways were the most up-regulated after treatment with α-LA. This finding, which has been confirmed by expression analyses of ER- and UPR-associated proteins, provides a better understanding of the molecular mechanisms behind the anti-tumoral action of α-LA on hepatoma cells. [4] Three self-assembled nanoaggregates (CPUL1-LA NAs, CPUL1-DA NAs, and CPUL1-AA NAs) were constructed through lipoic acid (LA), dithiodipropionic acid (DA), and adipic acid (AA) decorated TrxR inhibitor (CPUL1), respectively. Measurements of DLS, TEM, UV-vis, fluorescence, 1H NMR, ITC, and MTT assays verified disulfide-containing CPUL1-LA NAs and CPUL1-DA NAs spontaneously assembled carrier-free nanoparticles in aqueous solution, which possessed high drug contents, excellent stability, improved cytotoxicity against HUH7 hepatoma cells, and potential biosafety because of low cytotoxicity against L02 normal cells. In contrast, disulfide-free CPUL1-AA NAs happened to aggregate and precipitate after 48 h, which showed distinct instability in aqueous solution. Thus, disulfide units seemed to be crucial for constructing controllable and stable nanoaggregates. While measuring the reduction of nanoaggregates by TrxR/NADPH and GSH/GR/NADPH, cyclic disulfide of LA and linear disulfide of DA were verified to endow the nanoaggregates with targeting ability to respond specifically to TrxR over GSH. Furthermore, by tests of flow cytometry, fluorescence images, and CLSM, both CPUL1-LA NAs and CPUL1-DA NAs displayed a faster cellular uptake characteristic to be internalized by cancer cells and could generate more abundant ROS to induce cell apoptosis than that of free CPUL1, resulting in significantly improved antitumor efficacy against HUH7 cells in vitro [5]. |
| ln Vivo |
In order to cause nonalcoholic fatty liver disease (NAFLD), C57BL/6J mice are divided into four groups and fed a high-fat diet (HFD) for 24 weeks. Each group is then given daily administration of α-lipoic acid. The effects of α-Lipoic Acid are then examined in long-term HFD-fed mice with regard to hepatic lipid accumulation. Mice with visceral fat mass are significantly lessened after receiving 100 mg/kg or 200 mg/kg of -lipoic acid. Additionally, α-Lipoic Acid (100 mg/kg or 200 mg/kg) treatment reduces appetite and results in significant weight loss (all P0.05)[1].
Understanding the mechanism by which alpha-lipoic acid supplementation has a protective effect upon nonalcoholic fatty liver disease in vivo and in vitro may lead to targets for preventing hepatic steatosis. Male C57BL/6J mice were fed a normal diet, high-fat diet or high-fat diet supplemented with alpha-lipoic acid for 24 weeks. HepG2 cells were incubated with normal medium, palmitate or alpha-lipoic acid. The lipid-lowering effects were measured. The protein expression and distribution were analyzed by Western blot, immunoprecipitation and immunofluorescence, respectively. We found that alpha-lipoic acid enhanced sirtuin 1 deacetylase activity through liver kinase B1 and stimulated AMP-activated protein kinase. By activating the sirtuin 1/liver kinase B1/AMP-activated protein kinase pathway, the translocation of sterol regulatory element-binding protein-1 into the nucleus and forkhead box O1 into the cytoplasm was prevented. Alpha-lipoic acid increased adipose triacylglycerol lipase expression and decreased fatty acid synthase abundance. In in vivo and in vitro studies, alpha-lipoic acid also increased nuclear NF-E2-related factor 2 levels and downstream target amounts via the sirtuin 1 pathway. Alpha-lipoic acid eventually reduced intrahepatic and serum triglyceride content. The protective effects of alpha-lipoic acid on hepatic steatosis appear to be associated with the transcription factors sterol regulatory element-binding protein-1, forkhead box O1 and NF-E2-related factor 2 [3]. |
| Cell Assay |
The human hepatocellular carcinoma (HepG2) cell line is grown in Dulbecco's modified Eagle's medium at 37°C and 5% CO2 with 10% fetal bovine serum. The following substances are applied to HepG2 cells: AMPK inhibitor (CC, 20 μM, 0.5 h), SIRT1 inhibitor (NA, 10 mM, 12 or 24 h), AMPK activator (AICAR, 2 mM, 1 h), palmitate (PA, 125 μM, 12 h), and -Lipoic Acid (250 μM, 6 or 12 h)[1].
Cell lines [4] The rat hepatoma cell line, FaO, and the hepatocarcinoma cell line, HepG2, were maintained, respectively, in Dulbecco’s medium (DMEM plus Glutamax I) and supplemented with penicillin, streptomycin and 10% heat-inactivated fetal calf-serum (FCS) in a humidified atmosphere of 5% CO2/95% air, at 37 °C. α-lipoic acid (α-LA) and Thapsigargin (TG) were purchased from xxx. α-LA, dissolved in sodium hydroxide NaOH 1 N and neutralized in medium, and TG dissolved in DMSO, were added to the culture media to the final concentrations specified in the text. Morphological assessment of apoptosis [4] Morphological assessment and detection of apoptotic cells was performed using Hoechst 33258 staining. FaO cells (2 × 105 cells/well) were plated in chamber-slides and cultured in the presence or absence of α-LA/lipoic acid. After treatment, the cells were fixed with 2% paraformaldehyde and stained with Hoescht 33258. Stained cells were visualized under a Leica DM2000 fluorescence microscope and images were acquired with a digital camera Leica DCF420C. |
| Animal Protocol |
Mice: Male C57BL/6J mice (6 weeks old; body weight: 22-24 g) are divided into four groups (n=8) and given access to a normal diet and water ad libitum for two weeks. These groups are: normal diet (ND) (10% energy from fat), high-fat diet (HFD) (60% energy from fat), and HFD plus α-Lipoic Acid (100 mg/kg or 200 mg/kg). After the mice's eyes are removed for the preparation of the serum, blood samples are taken 24 weeks after the start of treatment. Centrifugation at 2000×g for 10 min. at 4 °C is used to separate the serum. Harvested in liquid nitrogen and kept at -80°C are the liver tissues.
Male C57BL/6J mice (6-week-old; body weight: 22–24 g) were housed in standard cage conditions at a constant temperature (22±1°C) and a 13:11-h light/dark cycle. All mice were allowed ad libitum access to normal diet and water for 2 weeks before dividing into four groups (n=8): normal diet (ND) (10% energy from fat; D12450B), high-fat diet (HFD) (60% energy from fat) and HFD plus ALA (100 mg/kg or 200 mg/kg). These doses of ALA/lipoic acid were selected to be similar to previous studies. After 24 weeks of treatment, blood samples were collected after the eyeballs of the mice were extracted for serum preparation by centrifugation at 2000×g for 10 min at 4°C. The liver tissues were harvested in liquid nitrogen and stored at −80°C. [3] |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
To determine the concentration of alpha-lipoic acid in the aqueous humour and investigate if its topical instillation can increase quantities. Methods: Seventy patients selected to undergo cataract surgery were randomly divided into two groups. Group 1 was used as a control group; for the patients in Group 2, a single instillation of alpha-lipoic acid eye drops (1%) was administered. Immediately before surgery an aliquot of 40-120 microL of aqueous humour was aspirated. The individual aspirations were combined to constitute pools representing time intervals with respect to administration. The levels of alpha-lipoic acid in the aqueous humour were measured using gas chromatography/mass-spectrometry. Pool 0 was created by combining the samples of aqueous humour obtained from the patients in Group 1, the control group, and the level of alpha-lipoic acid was 27.5 + 2.6 ng/mL; in the other pools the time interval between the administration of the eye drops and sampling was respectively 23 minutes, 53 minutes, 72 minutes, 93 minutes and 114 minutes, and the level of alpha-lipoic acid was 33.0 + 10.8 ng/mL; 52.0 + 2.5 ng/mL; 86.7 + 2.5 ng/mL; 91.2 + 2.5 ng/mL; 80.3 + 2.5 ng/mL. /The/ study demonstrates the presence of alpha-lipoic acid in the aqueous humour and indicates that its concentration increases after it is administered in the form of eye drops, reaching maximum values after around 93 minutes. The concentrations that are achieved in the anterior chamber allow us to theorise the possibility of exploiting the antioxidant properties of alpha-lipoic acid. R(+)-alpha-lipoic acid is a natural occurring compound that acts as an essential cofactor for certain dehydrogenase complexes. The redox couple alpha-lipoic acid/dihydrolipoic acid possesses potent antioxidant activity. Exogenous racemic alpha-lipoic acid orally administered for the symptomatic treatment of diabetic polyneuropathy is readily and nearly completely absorbed, with a limited absolute bioavailability of about 30% caused by high hepatic extraction. Although the pharmacokinetics of the parent drug have been well characterized in humans, relatively little is known regarding the excretion of alpha-lipoic acid and the pharmacokinetics of any metabolites in humans. In the present study, plasma concentration-time courses, urinary excreted amounts, and pharmacokinetic parameters of alpha-lipoic acid metabolites were evaluated in 9 healthy volunteers after multiple once-daily oral administration of 600 mg racemic alpha-lipoic acid. The primary metabolic pathways of alpha-lipoic acid in man, S-methylation and beta-oxidation, were quantitatively confirmed by an HPLC-electrochemical assay newly established prior to the beginning of this study. Major circulating metabolites were the S-methylated beta-oxidation products 4,6-bismethylthio-hexanoic acid and 2,4-bismethylthio-butanoic acid, whereas its conjugated forms accounted for the major portion excreted in urine. There was no statistically significant difference in the pharmacokinetic parameters Cmax, AUC, and tmax between day 1 and day 4. Despite the prolonged half-lives of the major metabolites compared to the parent drug, no evidence of accumulation was found. Mean values of 12.4% of the administered dose were recovered in the urine after 24 hours as the sum of alpha-lipoic acid and its metabolites. The results of the present study revealed that urinary excretion of alpha-lipoic acid and five of its main metabolites does not play a significant role in the elimination of alpha-lipoic acid. Therefore, biliary excretion, further electrochemically inactive degradation products, and complete utilization of alpha-lipoic acid as a primary substrate in the endogenous metabolism should be considered. In an open-label, parallel-group study involving 16 patients (8 with severely reduced renal function, 8 with end-stage renal disease needing hemodialysis), the effect of renal function on the pharmacokinetics, metabolism, and safety `of alpha-lipoic acid (thioctic acid) was evaluated by comparing the pharmacokinetic parameters with those of a reference group of 8 healthy subjects. Alpha-lipoic acid 600 mg was administered orally once daily for 4 days, and the pharmacokinetic parameters were measured on days 1 and 4. The mean percentage of the administered dose excreted in urine as parent compound was 0.2 and 0.05 in healthy subjects and subjects with severely reduced renal function, respectively. Assuming a bioavailability of 30%, this represents 0.67% and 0.17% of the bioavailable amount of alpha-lipoic acid, respectively. The percentage of total urinary recovered amounts of alpha-lipoic acid and 5 of its metabolites was 12.0 on both days. The respective values for patients with severe kidney damage were 5.2% (day 1) and 6.4% (day 4). The total percentage of the administered dose removed by hemodialysis was 4.0 in patients with end-stage renal disease. Renal clearance of alpha-lipoic acid and its major metabolites, 6,8-bismethylthio-octanoic acid, 4,6-bismethylthio-hexanoic acid and 2,4-bismethylthio-butanoic acid, were significantly decreased in subjects with kidney damage compared to the reference group. Apparent total clearance of alpha-lipoic acid was poorly correlated with creatinine clearance. There is strong evidence that alpha-lipoic acid is mainly excreted by nonrenal mechanism or further degraded to smaller units in the catabolic process. The significantly increased area under the curve values of 4,6-bismethylthio-hexanoic acid and half-lives of 2,4-bismethylthio-butanoic acid on both days in patients with severely reduced function and end-stage renal disease were not considered to be clinically relevant. Although trough levels of both metabolites tend to increase slightly in these subjects, no accumulation effects were detected. We conclude that the pharmacokinetics of alpha-lipoic acid are not influenced by creatinine clearance and are unaffected in subjects with severely reduced kidney function or end-stage renal disease. Hemodialysis did not significantly contribute to the clearance of alpha-lipoic acid. Hence, dose adjustment of alpha-lipoic acid is not necessary in patients with renal dysfunction. Alpha-lipoic aicd is absorbed from the small intestine and distributed to the liver via the portal circulation and to various tissues in the body via the systemic circulation.The natural R-enantiomer is more readily absorbed than the L-enantiomer and is the more active form. Alpha-lipoic acid readily crosses the blood-brain barrier. It is found, after its distribution to the various body tissues, intracellularly, intramitochondrialy and extracellularly. Metabolism / Metabolites Alpha-lipoic acid is metabolized to its reduced form, dihydrolipoic acid by mitochondrial lipoamide dehydrogenase. Dihydroipoic acid, together with lipoic acid, form a redox couple. It is also metabolized to lipoamide, which functions as the lipoic acid cofactor in the multienzyme complexes that catalyze the oxidative decarboxylations of pyruvate and alpha-ketoglutarate. Alpha-lipoic acid may be metabolized to dithiol octanoic acid, which can undergo catabolism. The excretion and biotransformation of rac-alpha-lipoic acid (LA), which is used for the symptomatic treatment of diabetic polyneuropathy, were investigated following single oral dosing of [(14)C]LA to mice (30 mg/kg), rats (30 mg/kg), dogs (10 mg/kg), and unlabeled LA to humans (600 mg). More than 80% of the radioactivity given was renally excreted. Metabolite profiles obtained by radiometric high-performance liquid chromatography revealed that LA was extensively metabolized irrespective of the species. Based on a new on-line liquid chromatography/tandem mass spectroscopy assay developed for negative ions, LA and a total of 12 metabolites were identified. Mitochondrial beta-oxidation played the paramount role in the metabolism of LA. Simultaneously, the circulating metabolites were subjected to reduction of the 1,2-dithiolane ring and subsequent S-methylation. In addition, evidence is given for the first time that the methyl sulfides formed were partly oxidized to give sulfoxides, predominantly in dogs. The disulfoxide of 2,4-bismethylmercapto-butanoic acid, the most polar metabolite identified, was the major metabolite in dogs. Furthermore, new data are presented that suggest conjugation with glycine occurred as a separate metabolic pathway in competition with beta-oxidation, predominantly in mice. |
| Toxicity/Toxicokinetics |
Toxicity Summary
ALA is generally considered a safe drug. A daily dose of 200 mg/day to 2400 mg/day of ALA is deemed safe without significant adverse effects. However, there is no reported safe dose in children. A notable case in literature demonstrated status epilepticus (SE) that subsided within a few days. The seizures were treated per normal standards for SE. In the last 2 decades, there have been few reported cases of ALA toxicity in humans. Most of these cases occur in children and are treatable. Though there is no established lethal dosage of ALA for humans, studies have shown that a high dose of 121 mg/kg body weight/day was associated with alterations in liver enzymes and liver function. Therefore, there are potentially harmful adverse effects from overdosing on ALA, and more studies are necessary to determine the toxicity. (R)-lipoic acid is a cholinesterase or acetylcholinesterase (AChE) inhibitor. A cholinesterase inhibitor (or 'anticholinesterase') suppresses the action of acetylcholinesterase. Because of its essential function, chemicals that interfere with the action of acetylcholinesterase are potent neurotoxins, causing excessive salivation and eye-watering in low doses, followed by muscle spasms and ultimately death. Nerve gases and many substances used in insecticides have been shown to act by binding a serine in the active site of acetylcholine esterase, inhibiting the enzyme completely. Acetylcholine esterase breaks down the neurotransmitter acetylcholine, which is released at nerve and muscle junctions, in order to allow the muscle or organ to relax. The result of acetylcholine esterase inhibition is that acetylcholine builds up and continues to act so that any nerve impulses are continually transmitted and muscle contractions do not stop. Among the most common acetylcholinesterase inhibitors are phosphorus-based compounds, which are designed to bind to the active site of the enzyme. The structural requirements are a phosphorus atom bearing two lipophilic groups, a leaving group (such as a halide or thiocyanate), and a terminal oxygen. Health Effects Acute exposure to cholinesterase inhibitors can cause a cholinergic crisis characterized by severe nausea/vomiting, salivation, sweating, bradycardia, hypotension, collapse, and convulsions. Increasing muscle weakness is a possibility and may result in death if respiratory muscles are involved. Accumulation of ACh at motor nerves causes overstimulation of nicotinic expression at the neuromuscular junction. When this occurs symptoms such as muscle weakness, fatigue, muscle cramps, fasciculation, and paralysis can be seen. When there is an accumulation of ACh at autonomic ganglia this causes overstimulation of nicotinic expression in the sympathetic system. Symptoms associated with this are hypertension, and hypoglycemia. Overstimulation of nicotinic acetylcholine receptors in the central nervous system, due to accumulation of ACh, results in anxiety, headache, convulsions, ataxia, depression of respiration and circulation, tremor, general weakness, and potentially coma. When there is expression of muscarinic overstimulation due to excess acetylcholine at muscarinic acetylcholine receptors symptoms of visual disturbances, tightness in chest, wheezing due to bronchoconstriction, increased bronchial secretions, increased salivation, lacrimation, sweating, peristalsis, and urination can occur. Certain reproductive effects in fertility, growth, and development for males and females have been linked specifically to organophosphate pesticide exposure. Most of the research on reproductive effects has been conducted on farmers working with pesticides and insecticdes in rural areas. In females menstrual cycle disturbances, longer pregnancies, spontaneous abortions, stillbirths, and some developmental effects in offspring have been linked to organophosphate pesticide exposure. Prenatal exposure has been linked to impaired fetal growth and development. Neurotoxic effects have also been linked to poisoning with OP pesticides causing four neurotoxic effects in humans: cholinergic syndrome, intermediate syndrome, organophosphate-induced delayed polyneuropathy (OPIDP), and chronic organophosphate-induced neuropsychiatric disorder (COPIND). These syndromes result after acute and chronic exposure to OP pesticides. Symptoms Symptoms of low dose exposure include excessive salivation and eye-watering. Acute dose symptoms include severe nausea/vomiting, salivation, sweating, bradycardia, hypotension, collapse, and convulsions. Increasing muscle weakness is a possibility and may result in death if respiratory muscles are involved. Hypertension, hypoglycemia, anxiety, headache, tremor and ataxia may also result. Treatment If the compound has been ingested, rapid gastric lavage should be performed using 5% sodium bicarbonate. For skin contact, the skin should be washed with soap and water. If the compound has entered the eyes, they should be washed with large quantities of isotonic saline or water. In serious cases, atropine and/or pralidoxime should be administered. Anti-cholinergic drugs work to counteract the effects of excess acetylcholine and reactivate AChE. Atropine can be used as an antidote in conjunction with pralidoxime or other pyridinium oximes (such as trimedoxime or obidoxime), though the use of '-oximes' has been found to be of no benefit, or possibly harmful, in at least two meta-analyses. Atropine is a muscarinic antagonist, and thus blocks the action of acetylcholine peripherally. |
| References |
|
| Additional Infomation |
Therapeutic Uses
/EXPERIMENTAL THERAPY/ The aim of this trial was to evaluate the effects of alpha-lipoic acid (ALA) on positive sensory symptoms and neuropathic deficits in diabetic patients with distal symmetric polyneuropathy (DSP). In this multicenter, randomized, double-blind, placebo-controlled trial, 181 diabetic patients in Russia and Israel received once-daily oral doses of 600 mg (n = 45) (ALA600), 1,200 mg (n = 47) (ALA1200), and 1,800 mg (ALA1800) of ALA (n = 46) or placebo (n = 43) for 5 weeks after a 1-week placebo run-in period. The primary outcome measure was the change from baseline of the Total Symptom Score (TSS), including stabbing pain, burning pain, paresthesia, and asleep numbness of the feet. Secondary end points included individual symptoms of TSS, Neuropathy Symptoms and Change (NSC) score, Neuropathy Impairment Score (NIS), and patients' global assessment of efficacy. Mean TSS did not differ significantly at baseline among the treatment groups and on average decreased by 4.9 points (51%) in ALA600, 4.5 (48%) in ALA1200, and 4.7 (52%) in ALA1800 compared with 2.9 points (32%) in the placebo group (all P < 0.05 vs. placebo). The corresponding response rates (> or = 50% reduction in TSS) were 62, 50, 56, and 26%, respectively. Significant improvements favoring all three ALA groups were also noted for stabbing and burning pain, the NSC score, and the patients' global assessment of efficacy. The NIS was numerically reduced. Safety analysis showed a dose-dependent increase in nausea, vomiting, and vertigo. CONCLUSIONS: Oral treatment with ALA for 5 weeks improved neuropathic symptoms and deficits in patients with DSP. An oral dose of 600 mg once daily appears to provide the optimum risk-to-benefit ratio. /EXPERIMENTAL THERAPY/ Mitochondria produce reactive oxygen species that may contribute to vascular dysfunction. alpha-Lipoic acid and acetyl-L-carnitine reduce oxidative stress and improve mitochondrial function. In a double-blind crossover study, the authors examined the effects of combined alpha-lipoic acid/acetyl-L-carnitine treatment and placebo (8 weeks per treatment) on vasodilator function and blood pressure in 36 subjects with coronary artery disease. Active treatment increased brachial artery diameter by 2.3% (P=.008), consistent with reduced arterial tone. Active treatment tended to decrease systolic blood pressure for the whole group (P=.07) and had a significant effect in the subgroup with blood pressure above the median (151+/-20 to 142+/-18 mm Hg; P=.03) and in the subgroup with the metabolic syndrome (139+/-21 to 130+/-18 mm Hg; P=.03). Thus, mitochondrial dysfunction may contribute to the regulation of blood pressure and vascular tone.... /EXPERIMENTAL THERAPY/ Lipoic acid is an antioxidant that suppresses and treats an animal model of multiple sclerosis, experimental autoimmune encephalomyelitis. The purpose of this study was to determine the pharmacokinetics (PK), tolerability and effects on matrix metalloproteinase-9 (MMP-9) and soluble intercellular adhesion molecule-1 (sICAMP-1) of oral lipoic acid in patients with multiple sclerosis. Thirty-seven MS subjects were randomly assigned to one of four groups: placebo, lipoic acid 600 mg twice a day, lipoic acid 1200 mg once a day and lipoic acid 1200 mg twice a day. Subjects took study capsules for 14 days. ... Subjects taking 1200 mg lipoic acid had substantially higher peak serum lipoic acid levels than those taking 600 mg and that peak levels varied considerably among subjects. We also found a significant negative correlation between peak serum lipoic acid levels and mean changes in serum MMP-9 levels (T = -0.263, P =0.04). There was a significant dose response relationship between lipoic acid and mean change in serum sICAM-1 levels (P =0.03). ... Oral lipoic acid is generally well tolerated and appears capable of reducing serum MMP-9 and sICAM-1 levels. Lipoic acid may prove useful in treating MS by inhibiting MMP-9 activity and interfering with T-cell migration into the CNS. /EXPERIMENTAL THERAPY/ Mitochondrial dysfunction and oxidative damage are highly involved in the pathogenesis of Parkinson's disease. Some mitochondrial antioxidants/nutrients that can improve mitochondrial function and/or attenuate oxidative damage have been implicated in Parkinson's disease therapy. However, few studies have evaluated the preventative effects of a combination of mitochondrial antioxidants/nutrients against Parkinson's disease, and even fewer have sought to optimize the doses of the combined agents. The present study examined the preventative effects of two mitochondrial antioxidant/nutrients, R-alpha-lipoic acid (LA) and acetyl-L-carnitine (ALC), in a chronic rotenone-induced cellular model of Parkinson's disease. We demonstrated that 4-week pretreatment with LA and/or ALC effectively protected SK-N-MC human neuroblastoma cells against rotenone-induced mitochondrial dysfunction, oxidative damage, and accumulation of alpha-synuclein and ubiquitin. Most notably, we found that when combined, LA and ALC worked at 100 to 1000 fold lower concentrations than they did individually. We also found that pretreatment with combined LA and ALC increased mitochondrial biogenesis and decreased production of reactive oxygen species through the upregulation of the peroxisome proliferator-activated receptor-gamma coactivator 1alpha as a possible underlying mechanism. This study provides important evidence that combining mitochondrial antioxidant/nutrients at optimal doses might be an effective and safe prevention strategy for Parkinson's disease. /R-alpha-lipoic acid/ For more Therapeutic Uses (Complete) data for alpha-Lipoic acid (11 total), please visit the HSDB record page. Drug Warnings Those with diabetes and problems with glucose intolerance are cautioned that supplemental alpha-lipoic acid may lower blood glucose levels. Blood glucose should be monitored and antidiabetic drug dose adjusted, if necessary, to avoid possible hypoglycemia. Because of lack of long-term safety data, alpha-lipoic acid should be avoided by pregnant and nursing mothers. The "Long Terminal Repeat" (LTR) of HIV-1 is the target of cellular transcription factors such as NF-kappaB, and serves as the promoter-enhancer for the viral genome when integrated in host DNA. Various LTR-reporter gene constructs have been used for in vitro studies of activators or inhibitors of HIV-1 transcription, e.g., to show that antioxidants such as lipoic acid and selenium inhibit NF-kappaB-dependent HIV-1 LTR activation. One such construct is the pHIVlacZ plasmid, with the HIV-1 LTR driving expression of the lacZ gene (encoding beta-galactosidase, beta-gal). Typically, for inhibitor screening, cells transfected with pHIVlacZ are activated using tumor necrosis factor-alpha (TNF-alpha), and the colorimetric o-nitrophenol assay is used to assess changes in beta-gal activity. A variant of this assay was developed as described here, in which LTR activation was induced by pro-fs, a novel HIV-1 gene product encoded via a -1 frameshift from the protease gene. Cotransfection of cells with pHIVlacZ along with a pro-fs construct produced a significant increase in beta-gal activity over controls. L-ergothioneine dose dependently inhibited both TNF-alpha-mediated and pro-fs-mediated increases in beta-gal activity, with an IC50 of about 6 mM. Thus antioxidant strategy involving ergothioneine derived from food plants might be of benefit in chronic immunodeficiency diseases.[1] Retinal ischemia-reperfusion (RIR) injury causes neuronal degeneration and initiates various optic nerve diseases. This study aimed to investigate the synergistic neuroprotective effect of rasagiline and idebenone against RIR injury. A combination of rasagiline and idebenone was administered intraperitoneally immediately after establishment of the RIR model. Treatment with the combination of the two drugs resulted in a significant restoration of retinal thickness and retinal ganglion cells. Apoptosis of cells in ganglion cell layers was also ameliorated, suggesting that the effect of the two drugs was synergistic and the expression of brain-derived neurotrophic factor increased. Furthermore, idebenone and rasagiline induced the expression of Lin28A and Lin28B, respectively, which resulted in a reduced expression of microRNAs in the let-7 family and an increased protein output of Dicer. The data obtained from gene overexpression and knockdown experiments indicated that let-7 and Dicer were necessary for the synergistic neuroprotective effect of the two drugs. Our findings suggested that combination therapy with rasagiline and idebenone produced a synergistic effect that ameliorated RIR injury and restored visual function. In addition, the combined treatment provided neuroprotection via enhancement of the selective regulation of let-7 by Lin28A/B. These findings implied that a treatment with the combination of rasagiline and idebenone is a feasible treatment option for optic nerve diseases.[2] |
| Molecular Formula |
C₈H₁₄O₂S₂
|
|---|---|
| Molecular Weight |
206.3256
|
| Exact Mass |
206.043
|
| Elemental Analysis |
C, 46.57; H, 6.84; O, 15.51; S, 31.08
|
| CAS # |
1077-28-7
|
| Related CAS # |
α-Lipoic Acid;1077-28-7
|
| PubChem CID |
864
|
| Appearance |
Light yellow to yellow solid powder
|
| Density |
1.2±0.1 g/cm3
|
| Boiling Point |
362.5±11.0 °C at 760 mmHg
|
| Melting Point |
60-62ºC
|
| Flash Point |
173.0±19.3 °C
|
| Vapour Pressure |
0.0±1.7 mmHg at 25°C
|
| Index of Refraction |
1.562
|
| LogP |
2.16
|
| Hydrogen Bond Donor Count |
1
|
| Hydrogen Bond Acceptor Count |
4
|
| Rotatable Bond Count |
5
|
| Heavy Atom Count |
12
|
| Complexity |
150
|
| Defined Atom Stereocenter Count |
0
|
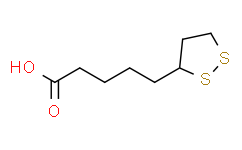
| SMILES |
S1C([H])(C([H])([H])C([H])([H])S1)C([H])([H])C([H])([H])C([H])([H])C([H])([H])C(=O)O[H]
|
| InChi Key |
AGBQKNBQESQNJD-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C8H14O2S2/c9-8(10)4-2-1-3-7-5-6-11-12-7/h7H,1-6H2,(H,9,10)
|
| Chemical Name |
5-(dithiolan-3-yl)pentanoic acid
|
| Synonyms |
Lipoic Acid; (R)-5-(1,2-Dithiolan-3-yl)pentanoic acid; R-(+)-alpha-Lipoic acid; (+)-alpha-Lipoic acid; Verla-Lipon; Lipoate; Verla Lipon; VerlaLipon; Thioctic Acid; Thioctacide T; Thiogamma Injekt; Thiogamma oral; thioctic acid; dl-Thioctic acid; 1077-28-7; alpha-Lipoic acid; lipoic acid; 5-(1,2-Dithiolan-3-yl)pentanoic acid; DL-alpha-Lipoic acid; 1,2-dithiolane-3-pentanoic acid;
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
DMSO: ~100 mg/mL (~484.7 mM)
H2O: < 0.1 mg/mL |
|---|---|
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples.
Injection Formulations
Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline)(e.g. IP/IV/IM/SC) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). View More
Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] Oral Formulations
Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). View More
Oral Formulation 3: Dissolved in PEG400 (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.8466 mL | 24.2330 mL | 48.4660 mL | |
| 5 mM | 0.9693 mL | 4.8466 mL | 9.6932 mL | |
| 10 mM | 0.4847 mL | 2.4233 mL | 4.8466 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03161028 | Active Recruiting |
Drug: Lipoic acid Drug: Placebo |
Multiple Sclerosis | VA Office of Research and Development |
July 1, 2018 | Phase 2 |
| NCT00765310 | Active Recruiting |
Dietary Supplement: R-alpha lipoic acid Dietary Supplement: Placebo |
Atherosclerosis | Oregon State University | April 2009 | Phase 2 Phase 3 |
| NCT00764270 | Active Recruiting |
Dietary Supplement: R-alpha lipoic acid |
Atherosclerosis | Oregon State University | August 2011 | Phase 2 Phase 3 |
| NCT02910531 | Active Recruiting |
Dietary Supplement: Alpha lipoic acid Drug: Placebo |
Cystinuria | Thomas Chi, MD | June 19, 2017 | Phase 2 |
| NCT02168140 | Active Recruiting |
Drug: bendamustine hydrochloride Drug: 6,8-bis(benzylthio)octanoic acid |
Peripheral T-cell Lymphoma Hepatosplenic T-cell Lymphoma |
Wake Forest University Health Sciences |
September 2014 | Phase 1 |