
| Size | Price | Stock | Qty |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
Purity: ≥98%
Vortioxetine lactate (formerly Lu-AA21004 lactate; Trintellix; Brintellix) is the lactate salt of Vortioxetine, which is an atypical antidepressant which was approved in 2013 by the FDA for the treatment of major depressive disorder (MDD) in adults. It has IC50 values of 15 nM, 33 nM, 3.7 nM, 19 nM, and 1.6 nM for 5-HT1A, 5-HT1B, 5-HT3A, 5-HT7 receptor, and SERT, respectively.
| Targets |
sPLA2 ( Ki = 15 nM ); 5-HT3A Receptor ( Ki = 3.7 nM ); 5-HT7 Receptor ( Ki = 19 nM ); SERT ( Ki = 1.6 nM )
|
||
|---|---|---|---|
| ln Vitro |
|
||
| ln Vivo |
|
||
| Enzyme Assay |
Vortioxetine (Compound 5m) is a multimodal serotonergic agent that inhibits SERT with values of 1.6 nM, 33 nM, 3.7 nM, 19 nM, and 5-HT1A, 5-HT1B, and 5-HT7 receptors, respectively. Vortioxetine exhibits strong suppression of SERT as well as antagonistic effects at 5-HT3A and 5-HT7 receptors, partial agonist effects at 5-HT1B receptors, and agonistic effects at 5-HT1A receptors.
Compound 5m (Lu AA21004) was the lead compound, displaying high affinity for recombinant human 5-HT(1A) (K(i) = 15 nM), 5-HT(1B) (K(i) = 33 nM), 5-HT(3A) (K(i) = 3.7 nM), 5-HT(7) (K(i) = 19 nM), and noradrenergic β(1) (K(i) = 46 nM) receptors, and SERT (K(i) = 1.6 nM). Compound 5m displayed antagonistic properties at 5-HT(3A) and 5-HT(7) receptors, partial agonist properties at 5-HT(1B) receptors, agonistic properties at 5-HT(1A) receptors, and potent inhibition of SERT.[1] Ex vivo SERT and 5-HT3 receptor occupancy assays[2] Brains from mice treated with vehicle, fluoxetine, or vortioxetine (1 h after acute administration or 24 h after the 14th or 21st injection) were flash frozen, sectioned coronally using a cryostat, and then mounted on slides and frozen until use. Slices were 20 μm thick, and began at approximately +1.2 mm anterior from bregma for SERT receptor occupancy or −2.7 mm posterior from bregma for 5-HT3 receptor occupancy determination (Franklin and Paxinos, 2008). Slides were stored for at least 24 h at −20 °C before use in autoradiography experiments. |
||
| Cell Assay |
Vortioxetine is a partial h5-HT1B receptor agonist that, in a whole-cell cAMP-based assay, has an EC50 of 460 nM and an intrinsic activity of 22%. In vitro whole-cell cAMP assay, vortioxetine binds to the r5-HT7 receptor with a Kivalue of 200 nM and is a functional antagonist at the r5-HT7 receptor with an IC50 of 2 μM.
Assessment of SERT occupancy[2] Slides were incubated at room temperature for 60 min in buffer (50 mM Tris–HCl, 150 mM NaCl, 5 mM KCl, pH = 7.4) containing 4.5 nM [3H]-escitalopram. Nonspecific binding was determined using 1 μM escitalopram. Slides were washed briefly in cold buffer, dried, and exposed in a Beta imager for 16 h. The region of interest (ROI) for the SERT assay included the lateral and medial septum, the nucleus accumbens and the olfactory tubercle. An example image of the ROI for the SERT assay can be found in Supplementary Fig. 2A. Assessment of 5-HT3 receptor occupancy[2] Slides were preincubated for 5 min in a buffer consisting of 50 mM Tris and 150 mM NaCl. Slides were dried under a stream of air for 30–45 min. Subsequently, slides were incubated at room temperature for 60 min in buffer (50 mM Tris–HCl, 150 mM NaCl, 5 mM KCl, pH = 7.4) containing 1 nM [3H]LY278584. Nonspecific binding was determined using 1 μM ondansetron. Slides were washed briefly in cold buffer, dried, and exposed in a Beta imager for 24 h. The ROI for the 5-HT3 receptor occupancy assay consisted of the hippocampus. An example image for the 5-HT3 receptor occupancy assay can be found in Supplementary Fig. 2B. |
||
| Animal Protocol |
|
||
| References |
|
||
| Additional Infomation |
Vortioxetine is an N-arylpiperazine in which the aryl group is specified as 2-[(2,4-dimethylphenyl)sulfanyl]phenyl. Used (as its hydrobromide salt) for treatment of major depressive disorder. It has a role as an antidepressant, an anxiolytic drug, a serotonergic agonist and a serotonergic antagonist. It is a N-arylpiperazine and an aryl sulfide. It is a conjugate base of a vortioxetine(1+).
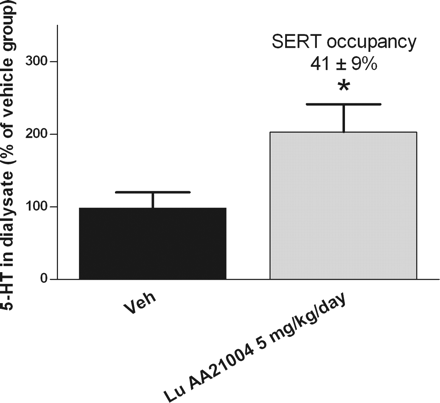
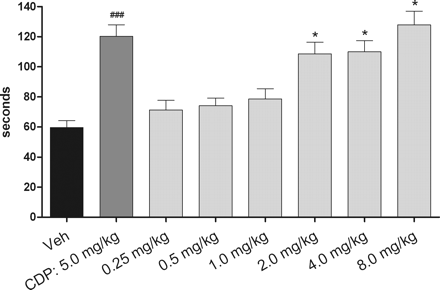
Vortioxetine is an antidepressant medication indicated for the treatment of major depressive disorder (MDD). It is classified as a serotonin modulator and stimulator (SMS) as it has a multimodal mechanism of action towards the serotonin neurotransmitter system whereby it simultaneously modulates one or more serotonin receptors and inhibits the reuptake of serotonin. More specifically, vortioxetine acts via the following biological mechanisms: as a serotonin reuptake inhibitor (SRI) through inhibition of the serotonin transporter, as a partial agonist of the 5-HT1B receptor, an agonist of 5-HT1A, and an antagonist of the 5-HT3, 5-HT1D, and 5-HT7 receptors. SMSs were developed because there are many different subtypes of serotonin receptors, however, not all of these receptors appear to be involved in the antidepressant effects of SRIs. Some serotonin receptors seem to play a relatively neutral or insignificant role in the regulation of mood, but others, such as 5-HT1A autoreceptors and 5-HT7 receptors, appear to play an oppositional role in the efficacy of SRIs in treating depression. Vortioxetine is a serotonergic antidepressant used for major depression disorders. Vortioxetine has been associated with a low rate of minor serum aminotransferase elevations during treatment, but has not been linked to instances of clinically apparent acute liver injury. A piperazine derivative that acts as a serotonin reuptake inhibitor, as a 5-HT3 receptor antagonist, and 5-HT1A receptor agonist. It is used for the treatment of anxiety and depression. Vortioxetine (Lu AA21004) is an investigational novel antidepressant with multimodal activity that functions as a 5-HT3, 5-HT7 and 5-HT(1D) receptor antagonist, 5-HT(1B) receptor partial agonist, 5-HT(1A) receptor agonist and inhibitor of the 5-HT transporter in vitro. Here we explore its anxiolytic and antidepressant potential in adult mice. Vortioxetine was assessed in BalB/cJ@RJ mice using the open-field and forced-swim tests (acute: p.o. 1 h, repeated: daily p.o. 21 days), and in 129S6/SvEvTac mice using the novelty suppressed feeding paradigm (acute: p.o. 1 h, sustained: daily p.o. 14 or 21 days). Fluoxetine and diazepam were controls. Acute and repeated dosing of vortioxetine produced more pronounced anxiolytic- and antidepressant-like activities than fluoxetine. Vortioxetine significantly increased cell proliferation and cell survival and stimulated maturation of immature granule cells in the subgranular zone of the dentate gyrus of the hippocampus after 21 days of treatment. After 14 days, a high dose of vortioxetine increased dendritic length and the number of dendrite intersections, suggesting that vortioxetine accelerates the maturation of immature neurons. Vortioxetine displays an antidepressant and anxiolytic profile following repeated administration associated with increased neurogenesis at several stages. Vortioxetine effects were observed at low levels of 5-HT transporter occupancy, suggesting an alternative mechanism of action to 5-HT reuptake inhibition.[2] View MoreThe aim of this study was to assess the effects of a novel antidepressant, vortioxetine 10 mg, on driving, cognitive, and psychomotor performance in 24 healthy subjects in a double-blind, placebo-controlled, three-way crossover design. Mirtazapine 30 mg was included as an active comparator. Drugs were administered in the evening of 15 consecutive days. Performance was measured in the morning of days 2 and 16, using standardized tests measuring on-the-road driving, memory, tracking, divided attention, and vigilance. The statistical analysis on the primary measure of driving, i.e., SD of lateral position showed noninferiority of vortioxetine on days 2 and 16, and inferiority for mirtazapine on day 2. Vortioxetine did not cause cognitive or psychomotor impairment. Mirtazapine, however, impaired cognitive and psychomotor performance on day 2. Most of these effects disappeared after multiple doses of mirtazapine. To conclude, vortioxetine did not impair driving, cognitive, or psychomotor performance after single or multiple doses.[3] Pharmacodynamics Vortioxetine binds with high affinity to the human serotonin transporter (Ki=1.6 nM), but not to the norepinephrine (Ki=113 nM) or dopamine (Ki>1000 nM) transporters. Vortioxetine potently and selectively inhibits reuptake of serotonin by inhibition of the serotonin transporter (IC50=5.4 nM). Specifically, vortioxetine binds to 5HT3 (Ki=3.7 nM), 5HT1A (Ki=15 nM), 5HT7 (Ki=19 nM), 5HT1D (Ki=54 nM), and 5HT1B (Ki=33 nM), receptors and is a 5HT3, 5HT1D, and 5HT7 receptor antagonist, 5HT1B receptor partial agonist, and 5HT1A receptor agonist. Absorption The maximal plasma vortioxetine concentration (Cmax) after dosing is reached within 7 to 11 hours postdose. Absolute bioavailability is 75%. No effect of food on the pharmacokinetics was observed. Route of Elimination Following a single oral dose of [14C]labeled vortioxetine, approximately 59% and 26% of the administered radioactivity was recovered in the urine and feces, respectively as metabolites. Negligible amounts of unchanged vortioxetine were excreted in the urine up to 48 hours. Volume of Distribution The apparent volume of distribution of vortioxetine is approximately 2600 L, indicating extensive extravascular distribution. Metabolism / Metabolites Vortioxetine is extensively metabolized primarily through oxidation via cytochrome P450 isozymes CYP2D6, CYP3A4/5, CYP2C19, CYP2C9, CYP2A6, CYP2C8 and CYP2B6 and subsequent glucuronic acid conjugation. CYP2D6 is the primary enzyme catalyzing the metabolism of vortioxetine to its major, pharmacologically inactive, carboxylic acid metabolite, and poor metabolizers of CYP2D6 have approximately twice the vortioxetine plasma concentration of extensive metabolizers. NIH; DailyMed. Current Medication Information for Brintellix (Vortioxetine Hydrobromide) Tablet, Film Coated (Updated: July 2014). Available from, as of June 30, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4b0700c9-b417-4c3a-b36f-de461e125bd3 All metabolites detected in human hepatocytes were also present in dogs, mice and rats (plasma and/or urine) in vivo, except for a glucuronide conjugate of monohydroxy-Vortioxetine which was not found in mice or rats. Among all species tested, rabbit hepatocytes appeared to have the metabolite profile closer to human hepatocyte metabolite profile. Biological Half-Life Mean terminal halflife is approximately 66 hours The oral absolute bioavailability was approximately 10% in the rat, 48% in the dog and 75% in patients, with terminal elimination half-life values of 3.0, 7.9 and 66 hours, respectively. Mechanism of Action Vortioxetine is classified as a serotonin modulator and simulator (SMS) as it has a multimodal mechanism of action towards the serotonin neurotransmitter system whereby it simultaneously modulates one or more serotonin receptors and inhibits the reuptake of serotonin. More specifically, vortioxetine acts via the following biological mechanisms: as a serotonin reuptake inhibitor (SRI) through inhibition of the serotonin transporter, while also acting as a partial agonist of the 5-HT1B receptor, an agonist of 5-HT1A, and antagonist of the 5-HT3, 5-HT1D, and 5-HT7 receptors. 1-(2-(2,4-Dimethylphenyl-sulfanyl)-phenyl)-piperazine (Lu AA21004) is a human (h) serotonin (5-HT)(3A) receptor antagonist (K(i) = 3.7 nM), h5-HT(7) receptor antagonist (K(i) = 19 nM), h5-HT(1B) receptor partial agonist (K(i) = 33 nM), h5-HT(1A) receptor agonist (K(i) = 15 nM), and a human 5-HT transporter (SERT) inhibitor (K(i) = 1.6 nM) (J Med Chem 54:3206-3221, 2011). Here, we confirm that Lu AA21004 is a partial h5-HT(1B) receptor agonist [EC(50) = 460 nM, intrinsic activity = 22%] using a whole-cell cAMP-based assay and demonstrate that Lu AA21004 is a rat (r) 5-HT(7) receptor antagonist (K(i) = 200 nM and IC(50) = 2080 nM). In vivo, Lu AA21004 occupies the r5-HT(1B) receptor and rSERT (ED(50) = 3.2 and 0.4 mg/kg, respectively) after subcutaneous administration and is a 5-HT(3) receptor antagonist in the Bezold-Jarisch reflex assay (ED(50) = 0.11 mg/kg s.c.). In rat microdialysis experiments, Lu AA21004 (2.5-10.0 mg/kg s.c.) increased extracellular 5-HT, dopamine, and noradrenaline in the medial prefrontal cortex and ventral hippocampus. Lu AA21004 (5 mg/kg per day for 3 days; minipump subcutaneously), corresponding to 41% rSERT occupancy, significantly increased extracellular 5-HT in the ventral hippocampus. Furthermore, the 5-HT(3) receptor antagonist, ondansetron, potentiated the increase in extracellular levels of 5-HT induced by citalopram. Lu AA21004 has antidepressant- and anxiolytic-like effects in the rat forced swim (Flinders Sensitive Line) and social interaction and conditioned fear tests (minimal effective doses: 7.8, 2.0, and 3.9 mg/kg). In conclusion, Lu AA21004 mediates its pharmacological effects via two pharmacological modalities: SERT inhibition and 5-HT receptor modulation. In vivo, this results in enhanced release of several neurotransmitters and antidepressant- and anxiolytic-like profiles at doses for which targets in addition to the SERT are occupied. The multimodal activity profile of Lu AA21004 is distinct from that of current antidepressants. The monoaminergic network, including serotonin (5-HT), norepinephrine (NE), and dopamine (DA) pathways, is highly interconnected and has a well-established role in mood disorders. Preclinical research suggests that 5-HT receptor subtypes, including 5-HT1A, 5-HT1B, 5-HT3, and 5-HT7 receptors as well as the 5-HT transporter (SERT), may have important roles in treating depression. This study evaluated the neuropharmacological profile of Lu AA21004, a novel multimodal antidepressant combining 5-HT3 and 5-HT7 receptor antagonism, 5-HT1B receptor partial agonism, 5-HT1A receptor agonism, and SERT inhibition in recombinant cell lines. Extracellular 5-HT, NE and DA levels were evaluated in the ventral hippocampus (vHC), medial prefrontal cortex (mPFC) and nucleus accumbens (NAc) after acute and subchronic treatment with Lu AA21004 or escitalopram. The acute effects of LuAA21004 on NE and DA neuronal firing were also evaluated in the locus coeruleus (LC) and ventral tegmental area (VTA), respectively. Acute Lu AA21004 dose-dependently increased 5-HT in the vHC, mPFC and NAc. Maximal 5-HT levels in the vHC were higher than those in the mPFC. Furthermore, mPFC 5-HT levels were increased at low SERT occupancy levels. In the vHC and mPFC, but not the NAc, high Lu AA21004 doses increased NE and DA levels. Lu AA21004 slightly decreased LC NE neuronal firing and had no effect on VTA DA firing. Results are discussed in context of occupancy at 5-HT3, 5-HT1B and 5-HT1A receptors and SERT. In conclusion, Lu AA21004, acting via two pharmacological modalities, 5-HT receptor modulation and SERT inhibition, results in a brain region-dependent increase of multiple neurotransmitter concentrations. and ECNP. All rights reserved. PMID:22612991 PMID:22612991 Pehrson AL et al; Eur Neuropsychopharmacol 23 (2): 133-45 (2013) Vortioxetine, a novel antidepressant with multimodal action, is a serotonin (5-HT)3, 5-HT7 and 5-HT1D receptor antagonist, a 5-HT1B receptor partial agonist, a 5-HT1A receptor agonist and a 5-HT transporter (SERT) inhibitor. Vortioxetine has been shown to improve cognitive performance in several preclinical rat models and in patients with major depressive disorder. Here we investigated the mechanistic basis for these effects by studying the effect of vortioxetine on synaptic transmission, long-term potentiation (LTP), a cellular correlate of learning and memory, and theta oscillations in the rat hippocampus and frontal cortex. Vortioxetine was found to prevent the 5-HT-induced increase in inhibitory post-synaptic potentials recorded from CA1 pyramidal cells, most likely by 5-HT3 receptor antagonism. Vortioxetine also enhanced LTP in the CA1 region of the hippocampus. Finally, vortioxetine increased fronto-cortical theta power during active wake in whole animal electroencephalographic recordings. In comparison, the selective SERT inhibitor escitalopram showed no effect on any of these measures. Taken together, our results indicate that vortioxetine can increase pyramidal cell output, which leads to enhanced synaptic plasticity in the hippocampus. Given the central role of the hippocampus in cognition, these findings may provide a cellular correlate to the observed preclinical and clinical cognition-enhancing effects of vortioxetine. Clinical Laboratory Methods In this work, a simple, sensitive and fast ultra performance liquid chromatography with tandem mass spectrometry (UPLC-MS/MS) method was developed and validated for the quantitative determination of vortioxetine in rat plasma. Plasma samples were processed with a protein precipitation. The separation was achieved by an Acquity UPLC BEH C18 column (2.1mmx50mm, 1.7um) column with a gradient mobile phase consisting of 0.1% formic acid in water and acetonitrile. Detection was carried out using positive-ion electrospray tandem mass spectrometry via multiple reaction monitoring (MRM). The validated method had an excellent linearity in the range of 0.05-20ng/mL (R2>0.997) with a lower limit of quantification (0.05ng/mL). The extraction recovery was in the range of 78.3-88.4% for vortioxetine and 80.3% for carbamazepine (internal standard, IS). The intra- and inter-day precision was below 8.5% and accuracy was from -11.2% to 9.5%. No notable matrix effect and astaticism was observed for vortioxetine. The method has been successfully applied to a pharmacokinetic study of vortioxetine in rats for the first time, which provides the basis for the further development and application of vortioxetine. PMID:26094207 Gu EM et al; J Chromatogr B Analyt Technol Biomed Life Sci 997: 70-74 (2015) Toxicity Summary IDENTIFICATION AND USE: Vortioxetine is a white to very slightly beige powder formulated into film-coated tablets. It is used for the management of major depressive disorders in adults. HUMAN EXPOSURE AND TOXICITY: There is limited clinical trial experience regarding human overdosage with vortioxetine. In pre-marketing clinical studies, cases of overdose were limited to patients who accidentally or intentionally consumed up to a maximum dose of 40 mg of vortioxetine. The maximum single dose tested was 75 mg in men. Ingestion of vortioxetine in the dose range of 40 to 75 mg was associated with increased rates of nausea, dizziness, diarrhea, abdominal discomfort, generalized pruritus, somnolence, and flushing. Toxicity may also occur at therapeutic dosage levels of vortioxetine. Potentially life-threatening serotonin syndrome has been reported with serotonergic antidepressants, including vortioxetine, when used alone, but particularly with concurrent use of other serotonergic drugs (including serotonin (5-hydroxytryptamine; 5-HT) type 1 receptor agonists ("triptans"), tricyclic antidepressants, buspirone, fentanyl, lithium, tramadol, tryptophan, and St. John's wort (Hypericum perforatum)) and with drugs that impair the metabolism of serotonin (particularly monoamine oxidase (MAO) inhibitors, both those used to treat psychiatric disorders and others, such as linezolid and methylene blue). Manifestations of serotonin syndrome may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, and hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, and incoordination), seizures, and/or GI symptoms (e.g., nausea, vomiting, and diarrhea). Concurrent or recent (i.e., within 2 weeks) therapy with MAO inhibitors intended to treat psychiatric disorders is contraindicated. Use of an MAO inhibitor intended to treat psychiatric disorders within 3 weeks of vortioxetine discontinuance also is contraindicated. Vortioxetine also should not be initiated in patients who are being treated with other MAO inhibitors such as linezolid or IV methylene blue. If concurrent therapy with vortioxetine and other serotonergic drugs is clinically warranted, the patient should be made aware of the potential increased risk for serotonin syndrome, particularly during initiation of therapy or when dosage is increased. Antidepressants increased the risk of suicidal thoughts and behavior in children, adolescents, and young adults in short-term studies. These studies did not show an increase in the risk of suicidal thoughts and behavior with antidepressant use in patients over age 24; there was a trend toward reduced risk with antidepressant use in patients aged 65 and older. Vortioxetine was not genotoxic in an in vitro chromosome aberration assay in cultured human lymphocytes. ANIMAL STUDIES: The acute oral single dose toxicity of vortioxetine is relatively low with a maximum tolerated dose (MTD) in mice and rats of 300 and 500 mg/kg, respectively. Clinical signs consisted of marked sensitivity to touch and disturbance, rapid breathing, and brown perinasal staining in rats administered 500 mg/kg. In mice, tremors, sensitivity to touch, eyes partly closed, and hypoactivity were seen after 200 and 300 mg/kg, as well as rapid, noisy and/or labored breathing, incoordination, unsteady gait, leaning, salivation, and hyperactivity after 400 and 500 mg/kg. When administered as two vortioxetine doses given an hour apart (200 mg/kg), clinical signs included convulsions, and resulted in death. Carcinogenicity studies were conducted in which mice and rats were given oral doses of vortioxetine up to 50 and 100 mg/kg/day for male and female mice, respectively, and 40 and 80 mg/kg/day for male and female rats, respectively, for 2 years. In rats, the incidence of benign polypoid adenomas of the rectum was statistically significantly increased in females. These were considered related to inflammation and hyperplasia and possibly caused by an interaction with a vehicle component of the formulation used for the study. The finding did not occur in male rats. In mice, vortioxetine was not carcinogenic in males or females. Vortioxetine caused developmental delays when administered during pregnancy to rats and rabbits. Developmental delays were also seen after birth in rats treated with vortioxetine during pregnancy and through lactation. There were no teratogenic effects in rats or rabbits treated with the drug during organogenesis. Treatment of rats with vortioxetine at doses up to 120 mg/kg/day had no effect on male or female fertility. Vortioxetine was not genotoxic in the in vitro bacterial reverse mutation assay (Ames test) and in the in vivo rat bone marrow micronucleus assay. Hepatotoxicity Liver test abnormalities occur in a small proportion of patients (Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury). Protein Binding The plasma protein binding of vortioxetine in humans is 98%, independent of plasma concentrations. No apparent difference in the plasma protein binding between healthy subjects and subjects with hepatic (mild, moderate) or renal (mild, moderate, severe, ESRD) impairment is observed. |
| Molecular Formula |
C21H28N2O3S
|
|
|---|---|---|
| Molecular Weight |
388.523624420166
|
|
| Exact Mass |
388.18
|
|
| CAS # |
1253056-29-9
|
|
| Related CAS # |
|
|
| PubChem CID |
75293775
|
|
| Appearance |
Typically exists as solid at room temperature
|
|
| Hydrogen Bond Donor Count |
3
|
|
| Hydrogen Bond Acceptor Count |
6
|
|
| Rotatable Bond Count |
4
|
|
| Heavy Atom Count |
27
|
|
| Complexity |
375
|
|
| Defined Atom Stereocenter Count |
0
|
|
| InChi Key |
KJXWEKCEAVJWHZ-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C18H22N2S.C3H6O3/c1-14-7-8-17(15(2)13-14)21-18-6-4-3-5-16(18)20-11-9-19-10-12-20;1-2(4)3(5)6/h3-8,13,19H,9-12H2,1-2H3;2,4H,1H3,(H,5,6)
|
|
| Chemical Name |
1-[2-(2,4-dimethylphenyl)sulfanylphenyl]piperazine;2-hydroxypropanoic acid
|
|
| Synonyms |
Vortioxetine lactate; Vortioxetine DL-lactate; Vortioxetine-DL-lactate; UNII-V39BK25ME9; V39BK25ME9; 1253056-29-9; Vortioxetine lactate [WHO-DD]; Q27291485
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples.
Injection Formulations
Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline)(e.g. IP/IV/IM/SC) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). View More
Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] Oral Formulations
Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). View More
Oral Formulation 3: Dissolved in PEG400 (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5739 mL | 12.8694 mL | 25.7387 mL | |
| 5 mM | 0.5148 mL | 2.5739 mL | 5.1477 mL | |
| 10 mM | 0.2574 mL | 1.2869 mL | 2.5739 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05814640 | Recruiting | Drug: Vortioxetine Drug: Duloxetine |
Depression Sequestra |
First Affiliated Hospital of Chongqing Medical University |
February 20, 2023 | Phase 1 Phase 2 |
| NCT04818099 | Recruiting | Drug: Vortioxetine 10 mg Other: Placebo |
Radiation Injuries | Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University |
October 10, 2020 | Phase 3 |
| NCT04301492 | Recruiting | Drug: Vortioxetine | Depression | IRCCS San Raffaele Roma | November 20, 2019 | Phase 4 |
| NCT02357797 | Active Recruiting |
Other: Placebo Drug: Vortioxetine |
Schizophrenia Negative Symptoms |
Northwell Health | February 2016 | Phase 4 |
| NCT06025474 | Recruiting | Drug: Vortioxetine 20Mg Tab Drug: Sertraline 50 MG |
Burning Mouth Syndrome | Federico II University | January 1, 2023 | Phase 3 |
 |
|---|
|
 |