
| Size | Price | Stock | Qty |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
Ropinirole (also known as AS-041164; SKF101468; Requip) a potent and selective dopamine D2 receptors agonist (Ki = 29 nM) of the non-ergoline class of medications with anti-PD effects. Ropinirole is mainly used for Parkinson's disease, RLS-restless legs syndrome and extrapyramidal symptoms. Additionally, it can lessen the side effects of selective serotonin reuptake inhibitors (SSRIs) and antipsychotics, such as Parkinsonism syndrome, erectile dysfunction, and sexual dysfunction. In the Fe2+–H2O2 reaction system, ropinirole scavenges free radicals and inhibits lipid peroxidation.
| Targets |
D2 Receptor ( Ki = 29 nM ); hD2 Receptor ( pEC50 = 7.4 ); hD3 Receptor ( pEC50 = 8.4 ); hD4.4 Receptor ( pEC50 = 6.8 )
|
|
|---|---|---|
| ln Vitro |
|
|
| ln Vivo |
|
|
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
Ropinirole is rapidly absorbed after oral administration, reaching peak concentration in approximately 1 to 2 hours,. Absolute bioavailability was 45% to 55%, suggesting approximately 50% hepatic first-pass effect. The bioavailability of ropinirole prolonged release compared to the immediate release tablets is about 100%. Ingestion of food does not affect the absorption of ropinirole, although its Tmax was increased by 2.5 hours and its Cmax was reduced by approximately 25% when the drug is taken with a high-fat meal. The majority of the absorbed dose is cleared by the liver. In clinical trials, more than 88% of a radiolabeled dose was recovered in urine. Less than 10% of the administered dose is excreted as unchanged drug in urine. _N-despropyl ropinirole_ is the major metabolite found in the urine (40%), followed by the _carboxylic acid_ metabolite (10%), and the _glucuronide_ of the hydroxy metabolite (10%). Ropinirole is found to be widely distributed throughout the body, with an apparent volume of distribution of **7.5 L/kg**. The clearance of ropinirole after oral administration is 47 L/h. The disposition and metabolic fate of ropinirole, a novel compound indicated for the symptomatic treatment of Parkinson's disease, was studied in the mouse, rat, cynomolgus monkey and man, following oral and intravenous administration of ropinirole hydrochloride. In all species, nearly all of the p.o. administered dose (94%) was rapidly absorbed from the gastrointestinal tract following administration of (14)C-ropinirole hydrochloride. In rat and monkey, the compound distributed rapidly beyond total body water and was shown to cross the blood-brain barrier. Blood clearance of the compound was high, approximately equal to one-half the hepatic blood flow in the monkey and similar to the hepatic blood flow in rat. Terminal phase elimination half-lives for the compound were relatively short (0.5 and 1.3 hr in rat and monkey respectively), although there was evidence of a second elimination phase in the monkey with an elimination half-life of approximately 5-11 hr. Plasma concentrations of ropinirole after the intravenous dose were not determined in the mouse and were below the lower limit of quantification in man (0.08 ng/mL) at the doses used in the studies described in this paper. In both animals and man, ropinirole was extensively metabolized. In the rat, the major metabolic pathway was via hydroxylation of the aromatic ring to form 7-hydroxy ropinirole. In mouse, monkey and man, the major pathway was via N-depropylation. The N-despropyl metabolite was metabolized further to form 7-hydroxy and carboxylic acid derivatives. Metabolites formed in all species were generally metabolized further by glucuronidation. 7-Hydroxy ropinirole is the only metabolite of ropinirole previously shown to possess significant dopamine agonist activity in vivo. In all species, the major route of excretion of ropinirole-related material after oral or intravenous administration of the compound was renal (60-90% of dose). Ropinirole is a selective non-ergoline dopamine D2 receptor agonist indicated for use in treating Parkinson's disease. When taken as oral tablets, ropinirole is rapidly and almost completely absorbed, and it is extensively distributed from the vascular compartment. The bioavailability is approximately 50%. Ropinirole shows low plasma protein binding. The drug is inactivated by metabolism in the liver, and none of the major circulating metabolites have pharmacological activity. The principal metabolic enzyme is the cytochrome P450 (CYP) isoenzyme CYP1A2. Ropinirole shows approximately linear pharmacokinetics when given as single or repeated doses, and is eliminated with a half-life of approximately 6 hours. Population pharmacokinetics have demonstrated that gender, mild or moderate renal impairment, Parkinson's disease stage and concomitant illnesses or the use of several common concomitant medications have no effect on the pharmacokinetics of ropinirole. Clearance is slower for patients older than 65 years compared with those who are younger, and in women taking hormone replacement therapy compared with those who are not. The CYP1A2 inhibitor ciprofloxacin produced increases in the plasma concentrations of ropinirole when these 2 drugs were coadministered, but no interaction was seen with theophylline which, like ropinirole, is also a substrate for CYP1A2. There is no obvious plasma concentration-effect relationship for ropinirole. Ropinirole is widely distributed throughout the body, with an apparent volume of distribution of 7.5 L/kg. It is up to 40% bound to plasma proteins and has a blood-to-plasma ratio of 1:1. Ropinirole is rapidly absorbed after oral administration, reaching peak concentration in approximately 1 to 2 hours. In clinical trials, more than 88% of a radiolabeled dose was recovered in urine and the absolute bioavailability was 45% to 55%, indicating approximately 50% first-pass effect. Relative bioavailability from a tablet compared with an oral solution is 85%. Food does not affect the extent of absorption of ropinirole, although its Tmax is increased by 2.5 hours and its Cmax is decreased by approximately 25% when the drug is taken with a high-fat meal. For more Absorption, Distribution and Excretion (Complete) data for ROPINIROLE (6 total), please visit the HSDB record page. Metabolism / Metabolites Ropinirole is heavily metabolized by the liver. The most important metabolic pathways are N despropylation and hydroxylation to form the _N-despropyl_ metabolite and _hydroxy_ metabolites, both of which are inactive. The _N-despropyl_ metabolite is then converted to _carbamyl glucuronide_, carboxylic acid, and _N-despropyl hydroxy_ metabolites. Following this process, the _hydroxy_ metabolite of ropinirole is glucuronidated at a rapid rate. _In vitro_ studies show that the major cytochrome P450 enzyme involved in the metabolism of ropinirole is CYP1A2,. Ropinirole is extensively metabolized by the liver. The N-despropyl metabolite is the major metabolite circulating in the plasma. Based on AUC data, the plasma levels of the metabolite were consistently higher than those of the parent drug suggesting a nonsaturable conversion of ropinirole to the N-despropyl metabolite. The affinity of the N-despropyl metabolite for human cloned D2 receptors is lower than the affinity of ropinirole. In addition the metabolite does not cross the blood-brain barrier; thus, it is unlikely to contribute to the therapeutic effects of ropinirole. The plasma concentrations of the hydroxylated metabolite are low and account for about 1-5% of the ropinirole concentrations. Although the hydroxylated metabolite was more active than ropinirole in in vitro D2 receptor binding studies, at therapeutic doses it is not expected to contribute to the activity of ropinirole. The disposition and metabolic fate of ropinirole, a novel compound indicated for the symptomatic treatment of Parkinson's disease, was studied in the mouse, rat, cynomolgus monkey and man, following oral and intravenous administration of ropinirole hydrochloride. ... In the rat, the major metabolic pathway was via hydroxylation of the aromatic ring to form 7-hydroxy ropinirole. In mouse, monkey and man, the major pathway was via N-depropylation. The N-despropyl metabolite was metabolized further to form 7-hydroxy and carboxylic acid derivatives. Metabolites formed in all species were generally metabolized further by glucuronidation. 7-Hydroxy ropinirole is the only metabolite of ropinirole previously shown to possess significant dopamine agonist activity in vivo. ... The dopamine receptor agonist ropinirole (SKF-101468) is used to treat Parkinson's disease. Ropinirole is metabolized by two routes to a series of different metabolites although the predominant pathway is species-dependent. It is unknown whether any of the metabolites contribute to its antiparkinsonian activity and whether D3 or D2 receptor agonist activity plays a preferential role. Therefore ropinirole and its primary metabolites, SKF-104557, SKF-97930 and SKF-96990, and the rat metabolite, SKF-89124 were tested in the 6-hydroxydopamine lesion model of Parkinson's disease. SKF-89124 and SKF-96990 were also assayed in radioligand binding and microphysiometer functional assays at cloned human dopamine D2 and D3. Ropinirole and SKF-89124 were equipotent in-vivo, and produced dose-related increases in circling at 0.05-0.8 mg kg(-1), s.c. (ropinirole) and 0.05-0.75 mg kg(-1), s.c. (SKF-89124). Neither SKF-96990 or SKF-97930, at doses up to 15 mg kg(-1), increased the circling rate. Some circling was observed with 15 mg kg(-1) SKF-104557 but the response was less than half that produced by ropinirole (0.8 mgkg(-1)). SKF-104557 was 150-fold less potent than ropinirole. SKF-89124 possessed-30-fold higher affinity for D3 over D2 receptors in radioligand binding studies, but was not selective in the functional microphysiometer assay. SKF-96990 was 10-fold selective for D3 over D2 receptors in the radioligand binding assay. Ropinirole and SKF-104557 are 20-fold selective for D3 over D2 receptors in radioligand binding assays whereas in microphysiometry, selectivity is 10-fold. SKF-97930 is inactive in radioligand binding and microphysiometer assays. Primary metabolites of ropinirole did not contribute significantly to its activity in this model of Parkinson's disease. The lack of dopamine D3/D2 receptor selectivity for ropinirole rules out the possibility of attributing the degree of either D2 or D3 receptor activity to the behavioural efficacy of ropinirole. The in vitro metabolism of ropinirole was investigated with the aim of identifying the cytochrome P450 enzymes responsible for its biotransformation. The pathways of metabolism after incubation of ropinirole with human liver microsomes were N-despropylation and hydroxylation. Enzyme kinetics demonstrated the involvement of at least two enzymes contributing to each pathway. A high affinity component with a K(M) of 5-87 uM and a low affinity component with a K(M) of approximately two orders of magnitude greater were evident. The high affinity component could be abolished by pre-incubation of the microsomes with furafylline. Additionally, incubation of ropinirole with microsomes derived from CYP1A2 transfected cells readily produced the N-despropyl and hydroxy metabolites. Some inhibition of ropinirole metabolism was also observed with ketoconazole, indicating a minor contribution by CYP3A. Multivariate correlation data were consistent with the involvement of the cytochrome P450 enzymes 1A2 and 3A in the metabolism of ropinirole. Thus, it could be concluded that the major P450 enzyme responsible for ropinirole metabolism at lower (clinically relevant) concentrations is CYP1A2 with a contribution from CYP3A, particularly at higher concentrations. Ropinirole has known human metabolites that include 4-[2-(Dipropylamino)ethyl]-7-hydroxy-1,3-dihydro-2h-indol-2-one and 4-(2-(Propylamino)ethyl)indolin-2-one. Hepatic. Ropinirole is extensively metabolized to inactive metabolites via N -despropylation and hydroxylation pathways, largely by the P450 isoenzyme CYP1A2. N-despropyl ropinirole is the predominant metabolite found in urine (40%), followed by the carboxylic acid metabolite (10%), and the glucuronide of the hydroxy metabolite (10%). Route of Elimination: Ropinirole is extensively metabolized by the liver to inactive metabolites, and less than 10% of the administered dose is excreted as unchanged drug in urine. Half Life: 6 hours Biological Half-Life Approximately 6 hours,. The terminal elimination half-life is approximately 6 hr (range 2-27 hr) ... . The disposition and metabolic fate of ropinirole, a novel compound indicated for the symptomatic treatment of Parkinson's disease, was studied in the mouse, rat, cynomolgus monkey and man, following oral and intravenous administration of ropinirole hydrochloride. ... Terminal phase elimination half-lives for the compound were relatively short (0.5 and 1.3 hr in rat and monkey respectively), although there was evidence of a second elimination phase in the monkey with an elimination half-life of approximately 5-11 hr. ... |
|
| Toxicity/Toxicokinetics |
Toxicity Summary
IDENTIFICATION AND USE: Ropinirole hydrochloride, a dipropylaminoethyl indolone derivative, is a nonergot-derivative dopamine receptor agonist. It is used for the symptomatic management of idiopathic parkinsonian syndrome. It is also used for the symptomatic management of moderate-to-severe primary restless legs syndrome (RLS).HUMAN EXPOSURE AND TOXICITY: The largest overdose reported with ropinirole in clinical trials was 435 mg taken over a 7-day period (62.1 mg/day). Of patients who received a dose greater than 24 mg/day, reported symptoms included adverse events commonly reported during dopaminergic therapy (nausea, dizziness), as well as visual hallucinations, hyperhidrosis, claustrophobia, chorea, palpitations, asthenia, and nightmares. Additional symptoms reported for doses of 24 mg or less or for overdoses of unknown amount included vomiting, increased coughing, fatigue, syncope, vasovagal syncope, dyskinesia, agitation, chest pain, orthostatic hypotension, somnolence, and confusional state. Postmarketing reports indicate that patients may experience new or worsening mental status and behavioral changes, which may be severe, including psychotic-like behavior during treatment with ropinirole or after starting or increasing the dose of ropinirole. This abnormal thinking and behavior can consist of one or more of a variety of manifestations including paranoid ideation, delusions, hallucinations, confusion, psychotic-like behavior, disorientation, aggressive behavior, agitation, and delirium. Case reports suggest that patients can experience intense urges to gamble, increased sexual urges, intense urges to spend money, binge or compulsive eating, and/or other intense urges, and the inability to control these urges while taking one or more of the medications, including ropinirole, that increase central dopaminergic tone and that are generally used for the treatment of Parkinson's disease and RLS. In some cases, although not all, these urges were reported to have stopped when the dose was reduced or the medication was discontinued. Ropinirole did not produced chromosomal aberration in human lymphocytes. ANIMAL STUDIES: Ropinirole has a biphasic effect on locomotor activity. Low doses inhibit spontaneous locomotion, while higher doses cause locomotor stimulation. In mice, 10 and 100 mg/kg ip doses brought about inhibition and stimulation, respectively. In rats, hypoactivity was observed at 0.3 mg/kg and hyperactivity in the 1-30 mg/kg dose range. Ropinirole induced a dose-related fall in blood pressure and reduced heart rate in anesthetized rats and in conscious spontaneously hypertensive rats. Single dose studies were performed in both mice and rats. The clinical signs were clearly dose-related and included hyperactivity, abnormal locomotion, stereotypy, tremors, convulsions and finally death. Two-year carcinogenicity studies of ropinirole were conducted in mice and in rats at oral doses of up to 50 mg/kg/day. In rats, there was an increase in testicular Leydig cell adenomas at all doses tested. In mice, there was an increase in benign uterine endometrial polyps at a dose of 50 mg/kg/day. The endocrine mechanisms involved in the production of these tumors in rats are not considered relevant to humans. Ropinirole was given to mated female rats. There were no maternal deaths or abortions. A dose related increase in post-implantation loss (up to 43%) and a decrease in mean fetal weight were noted. Retarded ossification of hindlimb metatarsals ant other malformations, including abnormal digits, neural tube defects and cardiovascular abnormalities were observed in the fetuses. Ropinirole was given at 0.1, 1.0 and 10 mg/kg/day to rats from day 15 of pregnancy to weaning. No maternal deaths or abortions were observed. While the weights of the high-dose pups was higher than that of the controls at age 1-2 days, their weight subsequently decreased and by day 14, they weighed 18% less than controls. The startle response to auditory and tactile stimulation was reduced in female, but not male offspring. When administered to female rats prior to and during mating and throughout pregnancy, ropinirole caused disruption of implantation. This effect in rats is thought to be due to the prolactin-lowering effect of ropinirole. In rat studies using a low oral dose (5 mg/kg) during the prolactin-dependent phase of early pregnancy, ropinirole did not affect female fertility at oral doses up to 100 mg/kg/day. No effect on male fertility was observed in rats at oral doses up to 125 mg/kg/day. Ropinirole was not mutagenic or clastogenic in in vitro (Ames, mouse lymphoma tk) assays or in the in vivo mouse micronucleus test. Ropinirole binds the dopamine receptors D3 and D2. Although the precise mechanism of action of ropinirole as a treatment for Parkinson's disease is unknown, it is believed to be related to its ability to stimulate these receptors in the striatum. This conclusion is supported by electrophysiologic studies in animals that have demonstrated that ropinirole influences striatal neuronal firing rates via activation of dopamine receptors in the striatum and the substantia nigra, the site of neurons that send projections to the striatum. Hepatotoxicity Ropinirole has been reported to cause serum aminotransferase or alkaline phosphatase elevations in a small proportion of patients, but these abnormalities are usually mild, asymptomatic and self-limiting even without dose adjustment. Ropinirole has been implicated in a small number of cases of acute liver injury, but the clinical characteristics and typical pattern of enzyme elevations has not been characterized. In one case report, the time to onset was 2 months and the pattern of liver enzyme elevations was mixed and associated with marked jaundice. Immunoallergic and autoimmune features were not present. The injury resolved within 2 months of stopping. Thus, ropinirole can cause acute, clinically apparent liver injury with jaundice, but it is rare. Likelihood score: D (possible, rare cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of ropinirole during breastfeeding, but it suppresses serum prolactin and may interfere with breastfeeding. An alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information on nursing mothers was not found as of the revision date. Ropinirole lowers serum prolactin. It has successfully been used clinically to reduce prolactin levels in women with hyperprolactinemia with relief of galactorrhea. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Protein Binding 40% bound to plasma proteins with a blood-to-plasma ratio of 1:1. Interactions Because ropinirole is a dopamine agonist, it is possible that dopamine antagonists such as neuroleptics (e.g., phenothiazines, butyrophenones, thioxanthenes) or metoclopramide may reduce the efficacy of Requip. Population pharmacokinetic analysis revealed that higher doses of estrogens (usually associated with hormone replacement therapy (HRT)) reduced the clearance of ropinirole. Starting or stopping HRT may require adjustment of dosage of Requip. No information is available on the potential for interaction between Requip and alcohol. As with other centrally active medications, patients should be cautioned against taking Requip with alcohol. The effect of Requip (2 mg t.i.d.) on the pharmacokinetics of digoxin (0.125-0.25 mg o.d.) was studied in male and female patients with Parkinson's disease (n=10, mean age 72 years). Coadministration at steady state with Requip resulted in a 10% decrease in digoxin AUC although mean trough digoxin plasma concentrations were unaltered. However, the effect of higher recommended doses of Requip on the pharmacokinetics of digoxin is not known. For more Interactions (Complete) data for ROPINIROLE (12 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat iv 66 mg/kg LD50 Rat po 862 mg/kg LD50 Mouse iv 46 mg/kg LD50 Mouse po 657 mg/kg |
|
| References | ||
| Additional Infomation |
Therapeutic Uses
Antiparkinson Agents; Dopamine Agonists /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health(NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Ropinirole is included in the database. Requip is indicated for the treatment of Parkinson's disease. /Included in US product label/ Requip is indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS). /Included in US product label/ For more Therapeutic Uses (Complete) data for ROPINIROLE (7 total), please visit the HSDB record page. Drug Warnings Postmarketing reports indicate that patients may experience new or worsening mental status and behavioral changes, which may be severe, including psychotic-like behavior during treatment with Requip or after starting or increasing the dose of Requip. Other drugs prescribed to improve the symptoms of Parkinson's disease can have similar effects on thinking and behavior. This abnormal thinking and behavior can consist of one or more of a variety of manifestations including paranoid ideation, delusions, hallucinations, confusion, psychotic-like behavior, disorientation, aggressive behavior, agitation, and delirium. Patients with a major psychotic disorder should ordinarily not be treated with Requip because of the risk of exacerbating the psychosis. In addition, certain medications used to treat psychosis may exacerbate the symptoms of Parkinson's disease and may decrease the effectiveness of Requip. Safety and effectiveness in pediatric patients have not been established. Ropinirole inhibits prolactin secretion in humans and could potentially inhibit lactation. Ropinirole has been detected in rat milk. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Requip is administered to a nursing woman. For more Drug Warnings (Complete) data for ROPINIROLE (21 total), please visit the HSDB record page. Pharmacodynamics **Effects on Parkinson's and restless leg syndrome** This drug promotes the relief or improvement of symptoms of Parkinson's or restless leg syndrome by stimulatory actions on dopamine receptors, which regulate movement. **Effects on blood pressure** Clinical experience with dopamine agonists, including ropinirole, suggests an association with impaired abilities in regulating blood pressure with resulting orthostatic hypotension, especially with patients undergoing dose escalation. In some patients in clinical studies, blood pressure changes were associated with orthostatic symptoms, bradycardia, and, in one case in a healthy volunteer, transient sinus arrest accompanied by syncope. The mechanism of orthostatic hypotension caused by ropinirole is assumed to be due to a D2-mediated blunting of noradrenergic response to a standing position, followed by a decrease in peripheral vascular resistance. Nausea is also a frequent symptom which accompanies orthostatic signs and symptoms. **Effects on prolactin** At oral doses as low as 0.2 mg, ropinirole suppressed serum prolactin concentrations in healthy male volunteers. Ropinirole had no dose-related effect on ECG wave form and rhythm in young, healthy, male volunteers in the range of 0.01 to 2.5 mg. **Effects on QT interval** Ropinirole had no dose- or exposure-related effect on average QT intervals in healthy male and female volunteers at doses up to 4 mg/day. The effect of ropinirole on QTc intervals at higher exposures reached either due to drug interactions, hepatic dysfunction, or at higher doses has not been adequately evaluated. |
| Molecular Formula |
C16H24N2O
|
|---|---|
| Molecular Weight |
260.37456
|
| Exact Mass |
260.188
|
| Elemental Analysis |
C, 73.81; H, 9.29; N, 10.76; O, 6.14
|
| CAS # |
91374-21-9
|
| Related CAS # |
Ropinirole hydrochloride; 91374-20-8; Ropinirole-d14; 1132746-05-4
|
| PubChem CID |
5095
|
| Appearance |
Solid powder
|
| Density |
1.0±0.1 g/cm3
|
| Boiling Point |
410.5±45.0 °C at 760 mmHg
|
| Melting Point |
243-250°C
|
| Flash Point |
202.0±28.7 °C
|
| Vapour Pressure |
0.0±1.0 mmHg at 25°C
|
| Index of Refraction |
1.539
|
| LogP |
3.19
|
| Hydrogen Bond Donor Count |
1
|
| Hydrogen Bond Acceptor Count |
2
|
| Rotatable Bond Count |
7
|
| Heavy Atom Count |
19
|
| Complexity |
287
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
O=C1CC2C(=CC=CC=2CCN(CCC)CCC)N1
|
| InChi Key |
UHSKFQJFRQCDBE-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C16H24N2O/c1-3-9-18(10-4-2)11-8-13-6-5-7-15-14(13)12-16(19)17-15/h5-7H,3-4,8-12H2,1-2H3,(H,17,19)
|
| Chemical Name |
4-[2-(dipropylamino)ethyl]-1,3-dihydroindol-2-one
|
| Synonyms |
Ropinirole; AS-041164; SKF 101468; AS 041164; SKF-101,468; AS041164; SKF 101468
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples.
Injection Formulations
Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline)(e.g. IP/IV/IM/SC) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). View More
Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] Oral Formulations
Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). View More
Oral Formulation 3: Dissolved in PEG400 (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.8407 mL | 19.2034 mL | 38.4069 mL | |
| 5 mM | 0.7681 mL | 3.8407 mL | 7.6814 mL | |
| 10 mM | 0.3841 mL | 1.9203 mL | 3.8407 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
Cognitive Remediation for Cocaine Dependence
CTID: NCT01393457
Phase: Phase 2 Status: Completed
Date: 2018-03-29
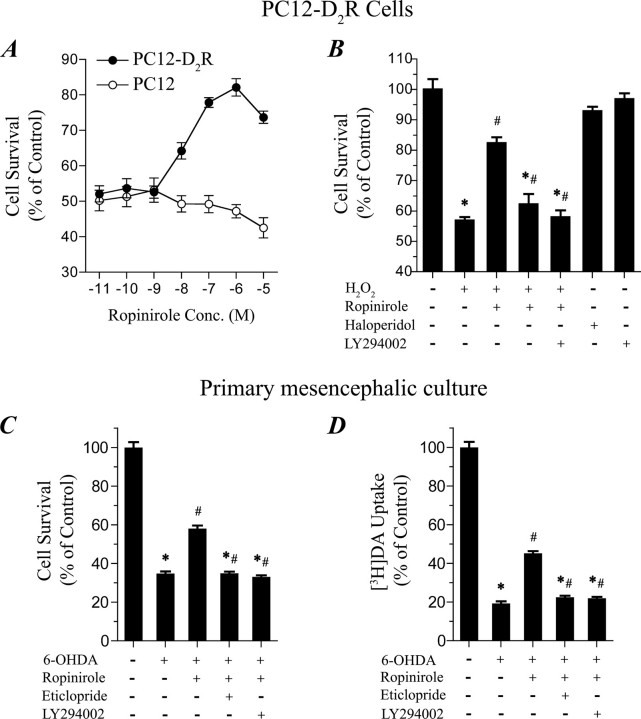 |
|---|
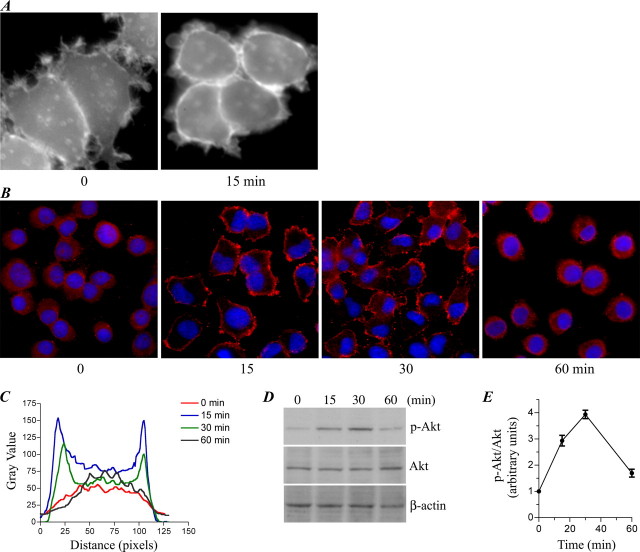 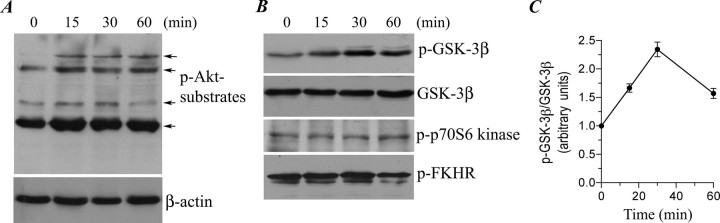 |
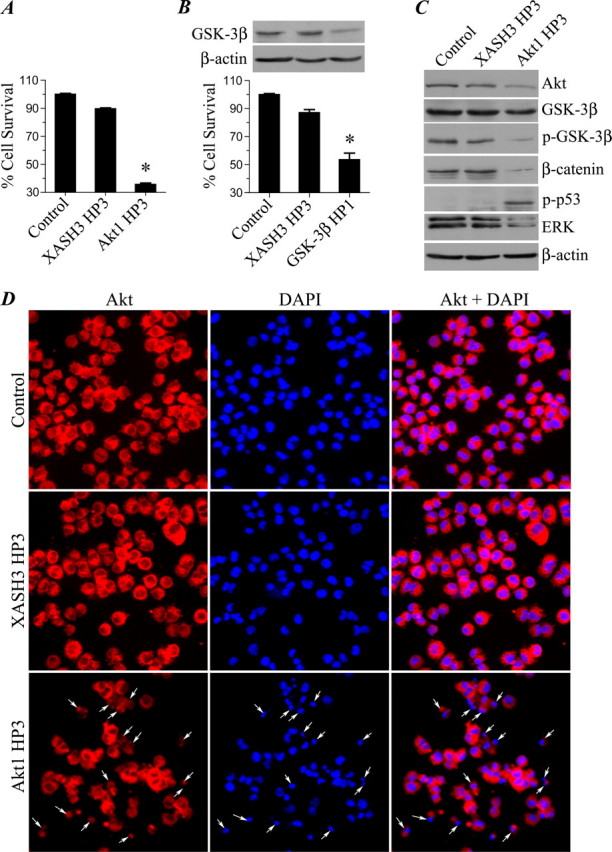 |