
| Size | Price | Stock | Qty |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
Purity: ≥98%
Nebivolol (R065824; R-065824; Nobiten; Vasoxen) is a potent and selective beta1/β1-adrenoceptor antagonist with antihypertensive effects. It suppresses the β1-adrenoceptor with an IC50 of 0.8 nM. Nebivolol is a beta blocker that has been used to treat heart failure and high blood pressure.
| ln Vitro |
Nebivolol has antioxidative and potent stimulatory effects on endothelial nitric oxide synthase activity, which improve endothelial dysfunction[1].
Nebivolol (0.1 μM–10 μM, 1, 2, 4, 7 or 14 days) inhibits the proliferation of haCSMC or haEC, with IC50 values of approximately 6.0 μM[2]. Nebivolol (0.1 μM-10 μM, 24 h) causes apoptosis at a moderate rate[2]. Nebivolol (0.1, 1, 5, 10 μM, 4 days) causes HaCEs to secrete less endothelin-1 and to produce more NO[2]. |
||
|---|---|---|---|
| ln Vivo |
|
||
| Cell Assay |
Nebivolol (10-7~10-5 M) is added to human coronary smooth muscle cells (haCSMCs) and endothelial cells (haECs) at varying concentrations for a period of 1, 2, 4, 7, and 14 days. Bromodeoxyuridine (BrdU) incorporation is used to analyze cell proliferation, while PI or annexin V staining is used to identify cell apoptosis.
|
||
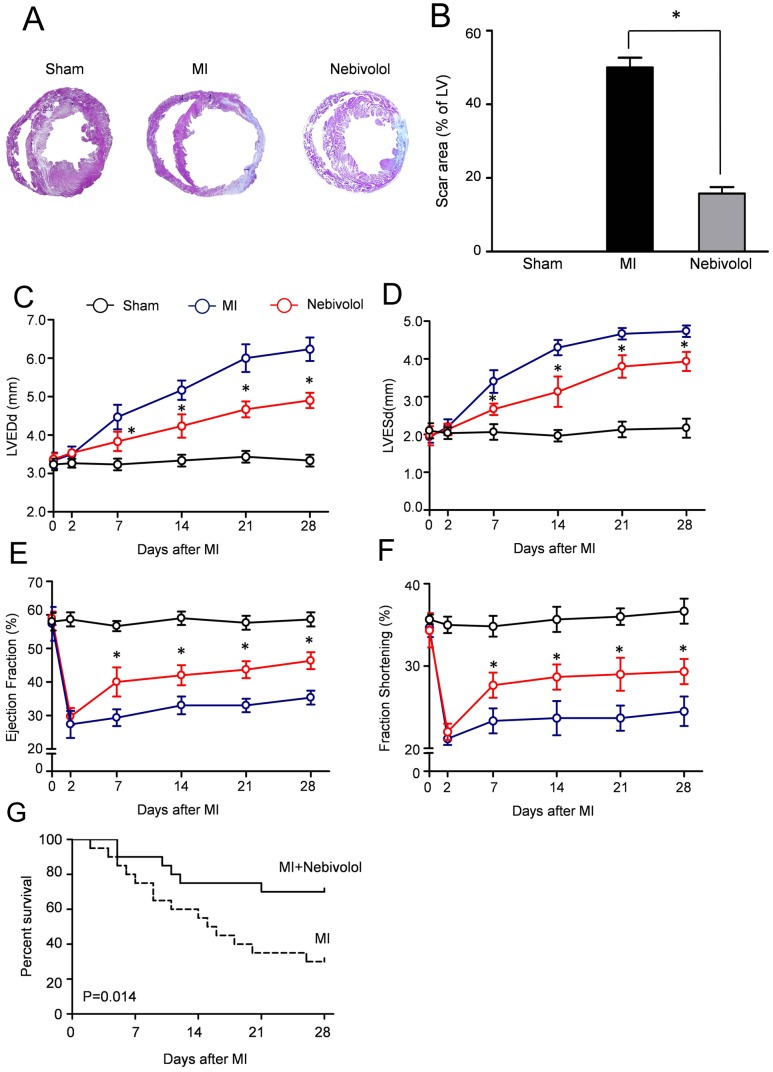
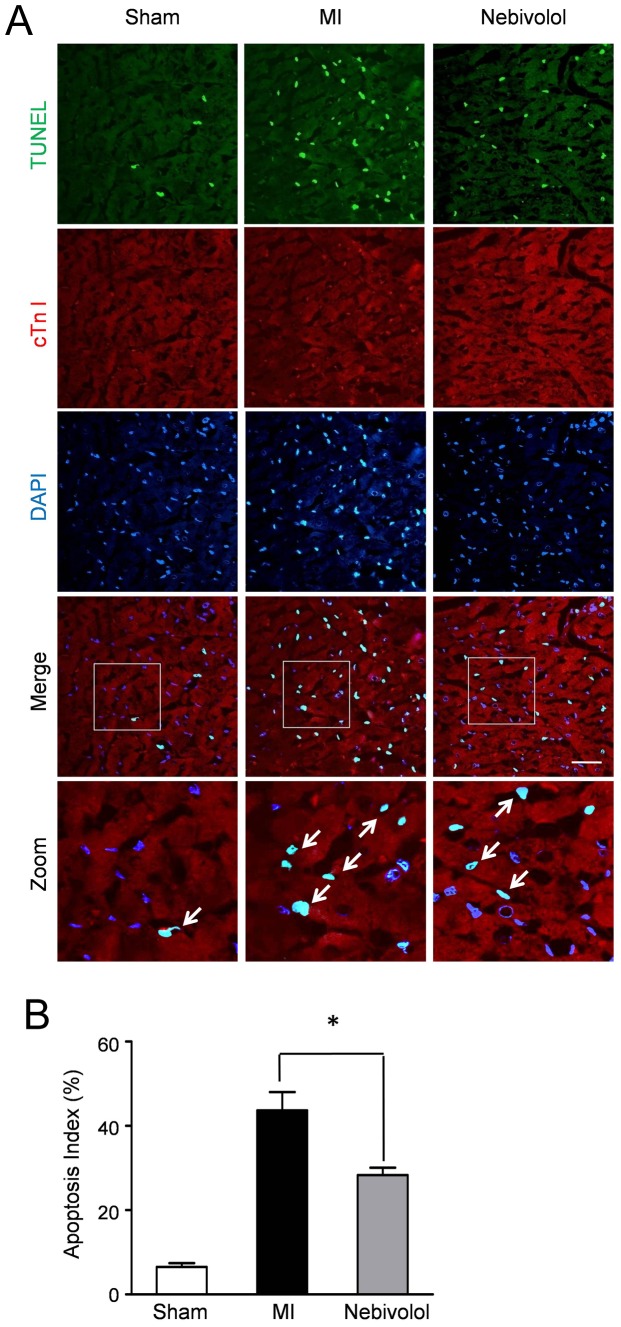
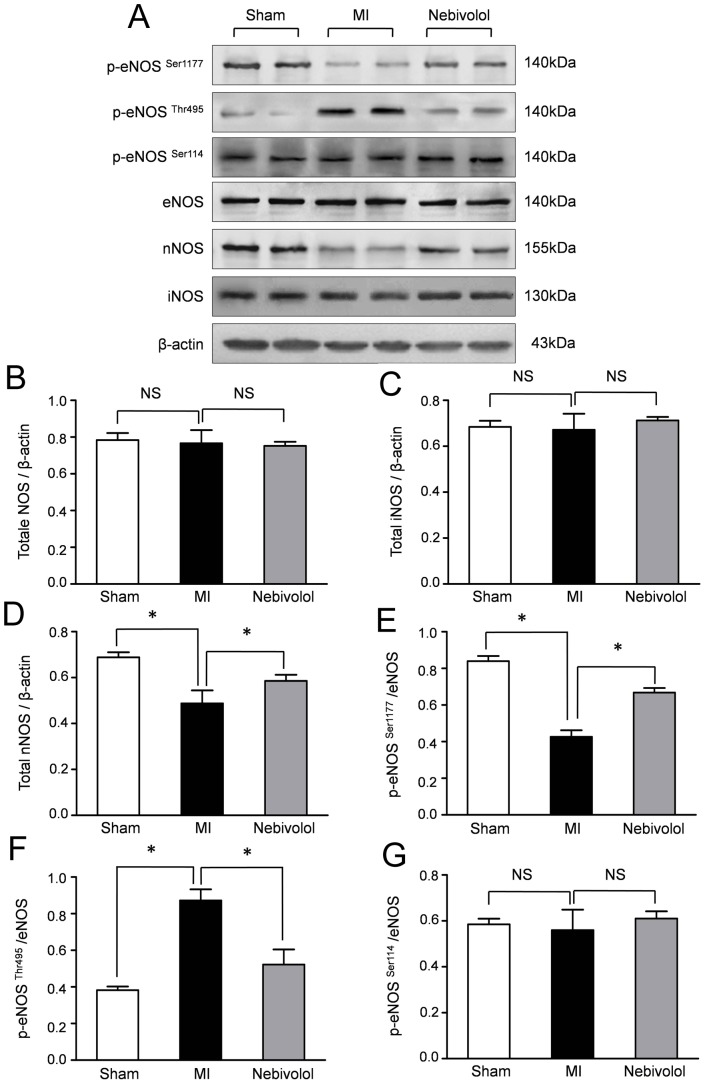
| Animal Protocol |
|
||
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
The absorption of nebivolol is not affected by food. Nebivolol has a Tmax of 1.5-4 hours. Bioavailability can range from 12-96% for extensive to poor CYP2D6 metabolizers. For a 20mg dose, d-nebivolol has a Cmax of 2.75±1.55ng/mL, l-nebivolol has a Cmax of 5.29±2.06ng/mL, both enantiomers have a Cmax of 8.02±3.47ng/mL, and nebivolol glucuronides have a Cmax of 68.34±44.68ng/mL. For a 20mg dose, d-nebivolol has an AUC of 13.78±15.27ng\*h/mL, l-nebivolol has an AUC of 27.72±15.32ng\*h/mL, both enantiomers have an AUC of 41.50±29.76ng\*h/mL, and nebivolol glucuronides have an AUC of 396.78±297.94ng\*h/mL. In extensive CYP2D6 metabolizers, 38% is eliminated in the urine and 44% in the feces. In poor CYP2D6 metabolizers, 67% is eliminated in the urine and 13% in the feces. <1% of a dose is excreted as the unmetabolized drug. For a 20mg dose, d-nebivolol has an apparent volume of distribution of 10,290.81±3911.72L, l-nebivolol has an apparent volume of distribution of 8,066.66±4,055.50L, and both enantiomers together have a volume of distribution of 10,423.42±6796.50L. For a 20mg dose, the clearance of d-nebivolol is 1241.63±749.77L/h, l-nebivolol is 435.53±180.93L/h, and both enantiomers is 635.31±300.25L/h. Metabolism / Metabolites Nebivolol is metabolized mainly by glucuronidation and CYP2D6 mediated hydroxylation. Metabolism involves n-dealkylation, hydroxylation, oxidation, and glucuronidation. Aromatic hydroxyl and acyclic oxide metabolites are active, while n-dealkylated and glucuronides are inactive. Biological Half-Life d-nebivolol has a half life of 12 hours in CYP2D6 extensive metabolizers and 19 hours in poor metabolizers. |
||
| Toxicity/Toxicokinetics |
Hepatotoxicity
Mild-to-moderate elevations in serum aminotransferase levels occur in less than 2% of patients on beta-blockers and are usually transient and asymptomatic, resolving even with continuation of therapy. There is no information on the rates of ALT elevations during nebivolol therapy. Despite its use in several large clinical trials, nebivolol has not been linked to cases of clinically apparent liver injury. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Because no information is available on the use of nebivolol during breastfeeding, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Nebivolol is 98% bound to plasma proteins, mostly to serum albumin. |
||
| References | |||
| Additional Infomation |
2,2'-iminobis[1-(6-fluoro-3,4-dihydro-2H-chromen-2-yl)ethanol] is a member of the class of chromanes that is 2,2'-iminodiethanol in which one hydrogen attached to each hydroxy-bearing carbon is replaced by a 6-fluorochroman-2-yl group. It is an organofluorine compound, a secondary amino compound, a secondary alcohol, a diol and a member of chromanes.
Nebivolol is a racemic mixture of 2 enantiomers where one is a beta adrenergic antagonist and the other acts as a cardiac stimulant without beta adrenergic activity. Treatment with nebivolol leads to a greater decrease in systolic and diastolic blood pressure than [atenolol], [propranolol], or [pindolol]. Nebivolol and other beta blockers are generally not first line therapies as many patients are first treated with thiazide diuretics. Nebivolol was granted FDA approval on 17 December 2007. Nebivolol is a beta-blocker and antihypertensive medication that has additional vasodilatory activity mediated by nitric oxide release. Nebivolol has yet to be linked to instances of clinically apparent liver injury. Nebivolol is a beta-1 adrenergic receptor antagonist with antihypertensive and vasodilatory activity. Nebivolol binds to and blocks the beta-1 adrenergic receptors in the heart, thereby decreasing cardiac contractility and rate. This leads to a reduction in cardiac output and lowers blood pressure. In addition, nebivolol potentiates nitric oxide (NO), thereby relaxing vascular smooth muscle and exerting a vasodilatory effect. A cardioselective ADRENERGIC BETA-1 RECEPTOR ANTAGONIST (beta-blocker) that functions as a VASODILATOR through the endothelial L-arginine/ NITRIC OXIDE system. It is used to manage HYPERTENSION and chronic HEART FAILURE in elderly patients. See also: Nebivolol Hydrochloride (has salt form). Drug Indication Nebivolol is indicated to treat hypertension. Mechanism of Action Nebivolol is a highly selective beta-1 adrenergic receptor antagonist with weak beta-2 adrenergic receptor antagonist activity. Blocking beta-1 adrenergic receptors by d-nebivolol leads to decreased resting heart rate, exercise heart rate, myocardial contracility, systolic blood pressure, and diastolic blood pressure. The selectivity of d-nebivolol limits the magnitude of beta blocker adverse effects in the airways or relating to insulin sensitivity. Nebivolol also inhibits aldosterone, and beta-1 antagonism in the juxtaglomerular apparatus also inhibits the release of renin. Decreased aldosterone leads to decreased blood volume, and decreased renin leads to reduced vasoconstriction. l-nebivolol is responsible for beta-3 adrenergic receptor agonist activity that stimulates endothelial nitric oxide synthase, increasing nitric oxide levels; leading to vasodilation, decreased peripheral vascular resistance, increased stroke volume, ejection fraction, and cardiac output. The vasodilation, reduced oxidative stress, and reduced platelet volume and aggregation of nebivolol may lead to benefits in heart failure patients. Pharmacodynamics Nebivolol is a selective beta-1 adrenergic receptor antagonist that decreases vascular resistance, increases stroke volume and cardiac output, and does not negatively affect left ventricular function. It has a long duration of action as effects can be seen 48 hours after stopping the medication and a wide therapeutic window as patients generally take 5-40mg daily. Patients should not abruptly stop taking this medication as this may lead to exacerbation of coronary artery disease. Diabetic patients should monitor their blood glucose levels as beta blockers may mask signs of hypoglycemia. |
| Molecular Formula |
C22H26CLF2NO4
|
|
|---|---|---|
| Molecular Weight |
441.9
|
|
| Exact Mass |
405.175
|
|
| Elemental Analysis |
C, 65.17; H, 6.22; F, 9.37; N, 3.45; O, 15.78
|
|
| CAS # |
118457-14-0
|
|
| Related CAS # |
Nebivolol hydrochloride; 152520-56-4; (Rac)-Nebivolol; 99200-09-6; (rac)-Nebivolol-d4; 1219407-55-2
|
|
| PubChem CID |
71301
|
|
| Appearance |
Solid powder
|
|
| Density |
1.3±0.1 g/cm3
|
|
| Boiling Point |
600.5±55.0 °C at 760 mmHg
|
|
| Melting Point |
223.0-228.0
|
|
| Flash Point |
316.9±31.5 °C
|
|
| Vapour Pressure |
0.0±1.8 mmHg at 25°C
|
|
| Index of Refraction |
1.581
|
|
| LogP |
3.67
|
|
| Hydrogen Bond Donor Count |
3
|
|
| Hydrogen Bond Acceptor Count |
7
|
|
| Rotatable Bond Count |
6
|
|
| Heavy Atom Count |
29
|
|
| Complexity |
483
|
|
| Defined Atom Stereocenter Count |
0
|
|
| SMILES |
FC1=CC=C2C(CC[C@]([C@@H](O)CNC[C@H](O)[C@@]3([H])CCC(C=C(F)C=C4)=C4O3)([H])O2)=C1
|
|
| InChi Key |
KOHIRBRYDXPAMZ-YHDSQAASSA-N
|
|
| InChi Code |
InChI=1S/C22H25F2NO4/c23-15-3-7-19-13(9-15)1-5-21(28-19)17(26)11-25-12-18(27)22-6-2-14-10-16(24)4-8-20(14)29-22/h3-4,7-10,17-18,21-22,25-27H,1-2,5-6,11-12H2/t17-,18-,21-,22+/m0/s1
|
|
| Chemical Name |
(1S)-1-[(2S)-6-fluoro-3,4-dihydro-2H-chromen-2-yl]-2-[[(2S)-2-[(2R)-6-fluoro-3,4-dihydro-2H-chromen-2-yl]-2-hydroxyethyl]amino]ethanol
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples.
Injection Formulations
Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline)(e.g. IP/IV/IM/SC) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). View More
Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] Oral Formulations
Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). View More
Oral Formulation 3: Dissolved in PEG400 (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2630 mL | 11.3148 mL | 22.6296 mL | |
| 5 mM | 0.4526 mL | 2.2630 mL | 4.5259 mL | |
| 10 mM | 0.2263 mL | 1.1315 mL | 2.2630 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
Determination of Drug Levels for Pharmacotherapy of Heart Failure
CTID: NCT06035978
Phase: Phase 4 Status: Not yet recruiting
Date: 2024-01-18
 |
|---|
 |
 |