
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| Other Sizes |
Purity: ≥98%
KN-93 Phosphate (KN 93; KN93), the phosphate salt of KN-93, is a potent, cell-permeable and specific inhibitor of Ca2+/calmodulin-dependent protein kinase II (CaMKII) with potential anti-Parkinson's disease and anticancer activity. It inhibits CaMKII with a Ki of 0.37 μM, and showed no effects on APK, PKC, MLCK or Ca2+-PDE activities. KN-93 suppresses ventricular arrhythmia induced by LQT2 without decreasing TDR. KN-93 inhibits androgen receptor activity and induces cell death irrespective of p53 and Akt status in prostate cancer. KN-93 ameliorates levodopa-induced dyskinesia in a rat model of Parkinson's disease. KN-93 protects rat cerebral cortical neurons from N-methyl-D-aspartic acid-induced injury.
| Targets |
CaMKII (calmodulin-dependent kinase type II) (Ki = 370 nM)
|
||
|---|---|---|---|
| ln Vitro |
Ninety-five percent of the cells were in the G1 phase after two days of treatment with KN-93 phosphate. G1 arrest is reversible, and cells peak into the S and G2-M phases one day after KN-93 phosphate is released. KN-93 phosphate also inhibits the proliferation of NIH 3T3 fibroblasts when they are stimulated by platelet-derived growth factor-BB, epidermal growth factor, and basic fibroblast growth factor [1]. While KN-93 phosphate strongly dissipates the proton gradient produced in stomach membrane vesicles and decreases cavity volume, it also inhibits the action of H+ and K+-ATPase [2]. Preventing LV developmental stress rises during action potential extension and early afterdepolarization is possible with KN-93 phosphate (0.5 μM). Early afterdepolarization is characterized by a rise in Ca2+-independent CaM kinase activity, which is inhibited by KN-93 phosphate [3].
Following two days of KN-93 treatment, 95% of cells exhibit G1 arrest. G1 arrest is reversible; a peak of cells had proceeded into S and G2-M one day after KN-93 release. In NIH 3T3 fibroblasts, KN-93 also inhibits the development of cells that are stimulated by basic fibroblast growth factor, platelet-derived growth factor-BB, and epidermal growth factor[1]. The H+, K+-ATPase activity is inhibited by KN-93, but the proton gradient created in the gastric membrane vesicles is strongly dissipated, and the luminal space volume is decreased[2]. Increased left ventricular developed pressure during action potential extension and early afterdepolarizations is prevented by KN-93 (0.5 μM). Early afterdepolarizations cause a rise in Ca2+-independent CaM kinase activity, which KN -93 blocks[3]. KN-93 (10 μM) dramatically suppresses the increased glucose-induced CaMKII/NF-κB signaling, which in turn reduces Müller cell production of VEGF, iNOS, and ICAM-1[4]. CaMK-II (the type II multifunctional Ca2+/calmodulin kinase) is a ubiquitous serine/threonine protein kinase that is activated by Ca2+ and calmodulin (CaM) and has been implicated in cell cycle control. NIH 3T3 fibroblast cytosolic extracts contain CaMK-II enzymatic activity and two major Ca2+/CaM-dependent phosphoproteins of M(r) 55,000 and 115,000. Reverse transcription-PCR indicates that the gamma B and gamma C isozymes of CaMK-II are predominately expressed. KN-93, a novel membrane-permeant synthetic inhibitor of purified neuronal CaMK-II, inhibits serum-induced fibroblast cell growth in a comparable dose-dependent fashion to its inhibition of CaMK-II activity. After 2 days of KN-93 treatment, 95% of cells are arrested in G1. G1 arrest is reversible; 1 day after KN-93 release, a peak of cells had progressed into S and G2-M. KN-92, a similar but inactive compound, had no effect on CaMK-II activity or cell growth. KN-93 also blocked cell growth stimulated by basic fibroblast growth factor, platelet-derived growth factor-BB, epidermal growth factor, and insulin-like growth factor-1. After 3 days of KN-93-induced G1 arrest, cell size and viability decreased and DNA fragmented, indicating apoptosis. These data suggest that CaMK-II is necessary for cell cycle progression through G1 and operates at a site common to the transduction of signals from growth and/or survival factors [1]. A novel Ca2+/calmodulin-dependent protein kinase II (CaM Kinase II) inhibitor, KN-93 potently inhibits gastric acid secretion from parietal cells. As previously reported (1), treatment of parietal cells with a selective inhibitor of CaM kinase II, KN-62 resulted in the inhibition of cholinergic-stimulated rabbit parietal cell secretion, whereas it failed to inhibit the histamine and forskolin response. In contrast effects of carbachol, histamine and forskolin were significantly inhibited by KN-93 with an IC50 of 0.15, 0.3 and 1 microM, respectively; these effects occurred without any changes in intracellular cyclic AMP and Ca2+ levels. In the present study we investigated the mechanism by which KN-93 acts upon the acid-secreting machinery of gastric parietal cells. Neither redistribution of the proton pump activity nor the morphological transformation were affected by KN-93. The drug only weakly inhibited the H+, K(+)-ATPase activity but strongly dissipated the proton gradient formed in the gastric membrane vesicles and reduced the volume of luminal space. Thus KN-93 acts at pH gradient formation whereas KN-62 acts only at CaM Kinase II [2]. |
||
| ln Vivo |
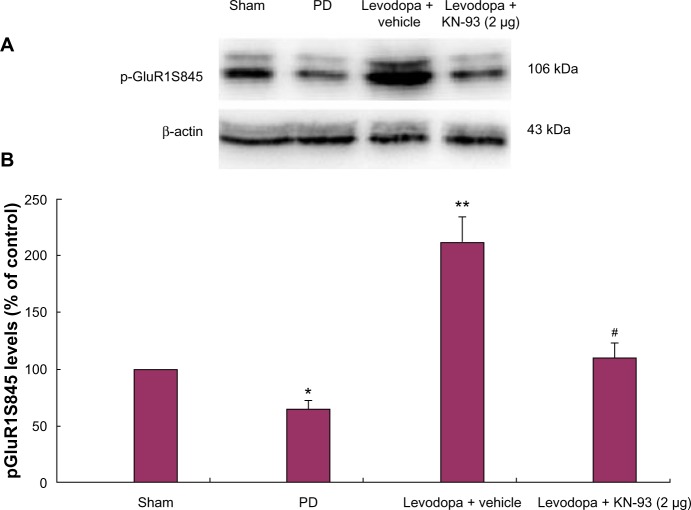
KN-93 (5 μg) ameliorates levodopa-induced dyskinesia by lowering the expression of pGluR1S845 in a rat model of Parkinson’s disease. In MRL/lpr Foxp3-GFP mice, KN-93 results in a significant induction of Treg cells in the spleen, peripheral lymph nodes and peripheral blood, and decreases skin and kidney damage.
KN-93 (1 mg/kg/day, ip) decreases phosphorylation of CaMKII and NF-κB in diabetic retina and inhibits retinal vascular leakage caused by diabetes[4]. The multifunctional Ca++/calmodulin-dependent protein kinase II (CaM kinase) mediates Ca++-induced augmentation of L-type Ca++ current (ICa); therefore it may act as a proarrhythmic signaling molecule during early afterdepolarizations (EADs) due to ICa. To investigate the hypothesis that ICa-dependent EADs are favored by CaM kinase activation EADs were induced with clofilium in isolated rabbit hearts. All EADs were rapidly terminated with ICa antagonists. Hearts were pretreated with the CaM kinase inhibitor KN-93 or the inactive analog KN-92 (0.5 microM) for 10 min before clofilium exposure. EADs were significantly suppressed by KN-93 (EADs present in 4/10 hearts) compared to KN-92 (EADs present in 10/11 hearts) (P =.024). There were no significant differences in parameters favoring EADs such as monophasic action potential duration or heart rate in KN-93- or KN-92-treated hearts. CaM kinase activity in situ increased 37% in hearts with EADs compared to hearts without EADs (P =.015). This increase in CaM kinase activity was prevented by pretreatment with KN-93. In vitro, KN-93 potently inhibited rabbit myocardial CaM kinase activity (calculated Ki 100 microM). The actions of KN-93 and KN-92 on ICa and other repolarizing K+ currents did not explain preferential EAD suppression by KN-93. These data show a novel association between CaM kinase activation and EADs and are consistent with the hypothesis that the ICa and CaM kinase activation both contribute to EADs in this model. [3] Curcumin and KN-93 inhibit retinal vascular leakage induced by diabetes [4] Evans blue was used in retinal flat mounts to evaluate the effect of curcumin on retinal blood vessel leakage. In control retinas, Evans blue fluorescence was located within blood vessels (Fig. 2A). In STZ-treated rats, focal leakage of the dye from capillaries and larger vessels was noted (Fig. 2B, arrows), in agreement with other reports. This leakage was not seen in STZ-treated rats which were administered curcumin (Fig. 2C) or KN93 (Fig. 2D). Evans blue in the retina was measured to assess BRB permeability (Fig. 2E). Evans blue levels were elevated in the retinas of STZ-treated diabetic rats (2.89±0.47 μg Evans blue/g wet wt retina) as compared to control animals (0.82±0.11 μg Evans blue/g wet wt retina). In agreement with the reduced vascular leakage indicating by whole mount imaging, this elevation was significantly reduced in STZ-treated rats administered curcumin (1.24±0.21 μg) or KN93 (1.37±0.35 μg). Curcumin and KN-93 reduce VEGF, iNOS and ICAM-1expession in the diabetic retina [4] Vscular leakage and adhesion of leukocytes to retinal vessels is mediated by pro-inflammatory cytokines. Therefore, the effect of curcumin on the expression levels of VEGF, iNOS and ICAM-1 was measured. In comparison to non-diabetic controls, mRNA (Fig. 3A) and protein (Fig. 3B, C) measures were significantly elevated in the retina of STZ-treated diabetic rats. These increases were significantly reduced by administration of curcumin or KN93. Curcumin and KN-93 suppress phosphorylation of CaMKII and NF-κB in diabetic retina [4] Phosphorylation of the p65 subunit of NF-κB plays an important role in regulating the expression of many genes, including those that encode pro-inflammatory cytokines and adhesion molecules. In addition, phosphorylation of CaMKII is a critical factor in the development of retinal vascular damage in diabetic mice. To evaluate the role of curcumin in the regulation of the phosphorylation of CaMKII and NF-κB p65, we examined retinas by Western blot. As shown in Figure 5, levels of phosphorylated CaMKII (Thr286) and NF-kB p65 (Ser536) were significantly elevated in retinas of STZ-treated diabetic rats as compared to controls. This elevation was normalized in STZ-treated diabetic rats that were administered curcumin (100 mg/kg/day) or KN93 (1 mg/kg/day). Both 2 μg and 5 μg KN-93 treatment lowered AIMs scores in levodopa priming PD rats without affecting the antiparkinsonian effect of levodopa. In agreement with behavioral analysis, KN-93 treatment (2 μg) reduced pGluR1S845 levels in PD rats. Moreover, KN-93 treatment (2 μg) reduced the expression of Gad1 and Nur77 in PD rats. Conclusion: These data indicated that intrastriatal injections of KN-93 were beneficial in reducing the expression of LID by lowering the expression of pGluR1S845 via suppressing the activation of CaMKII in PD rats. Decreased expression of pGluR1S845 further reduced the expression of Gad1 and Nur77 in PD rats [5]. |
||
| Cell Assay |
For primary culture studies, rat retinal Müller cells were obtained and identified as described previously. Briefly, Sprague-Dawley rats at postnatal (PN) day 5 to PN7 were sacrificed and the enucleated eyes were washed under sterile conditions, and the anterior portions were discarded. The retinas were isolated, chopped into 1×1 mm fragments, treated with 0.1% trypsin at 37°C for 20 min, and then passed through mesh to remove any large retinal pieces. The strained isolates were centrifuged at 800 rpm for 5 min, and the supernatant fluid was removed. The precipitated cells were resuspended and seeded onto plastic culture flasks containing Dulbecco's modified Eagle's medium (DMEM) supplemented with 2 mmol/L glutamine, 0.1% penicillin/streptomycin and 10% fetal calf serum. The cultures were maintained in 5% CO2 at 37°C. The medium was routinely replaced every 3-4 d. Müller cells were identified by their expression of glutamine synthetase (GS) and vimentin, as judged by immunocytochemical staining. Nuclei were stained with DAPI (4',6-diamidino-2-phenylindole). All experiments were conducted using 80%-85% confluent cells. Before each experiment, the plated cells were incubated with serum-free DMEM medium for 1 h. After this, the medium was replaced with serum-free DMEM and the cells were treated with normal D-glucose (5.5 mmol/L) or high glucose (HG; 30 mmol/l glucose) in the presence or absence of 10 μmol/L KN-93, 100 μmol/L PDTC (pyrrolidine dithiocarbamate, a NF-kB inhibitor), or curcumin at the indicated concentrations. [4]
Evaluation of Cell Viability: Cell viability was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) assay. Briefly, Müller cells were seeded at a density of 10×104 cells per well in 96-well plates and cultured until sub-confluence. Next, cells were treated with curcumin for 24 h before incubation with MTT (5 mg/mL) at 37°C in 5% CO2 atmosphere for 4 h. The culture medium was then removed, and the formazan formed in the reaction was dissolved in 150 μL DMSO (dimethyl sulphoxide). The optical density of the solution was measured at 490 nm using a multifunctional microplate reader. Cell viability in each well was presented as a percentage of the control (vehicle-treated group). |
||
| Animal Protocol |
|
||
| References | |||
| Additional Infomation |
KN-93 is a sulfonamide resulting from the formal condensation of p-methoxybenzenesulfonic acid with the anilino nitrogen of 2-(aminomethyl)-N-(2-hydroxyethyl)aniline in which the hydrogens of the primary amino group have been replaced by methyl and p-chlorocinnamyl groups. KN-93 is a selective inhibitor of Ca(2+)/calmodulin-dependent protein kinase II. It has a role as an EC 2.7.11.17 (Ca(2+)/calmodulin-dependent protein kinase) inhibitor and a geroprotector. It is a sulfonamide, a tertiary amino compound, a primary alcohol, a member of monochlorobenzenes and a monomethoxybenzene.
Background: Levodopa remains the most effective drug for the treatment of Parkinson's disease (PD). However, long-term levodopa treatment is associated with the emergence of levodopa-induced dyskinesia (LID), which has hampered its use for PD treatment. The mechanisms of LID are only partially understood. A previous study showed that KN-93, a Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) inhibitor, could be used to ameliorate LID in rats. However, the precise mechanisms by which KN-93 acts as an antidyskinetic are not fully understood. Methods: In the present study, a rat model of PD was induced by 6-hydroxydopamine (OHDA) injections. Then, the successfully lesioned rats were intrastriatally administered with a different dose of KN-93 (1 μg, 2 μg, or 5 μg) prior to levodopa treatment. Abnormal involuntary movements (AIMs) scores and apomorphine-induced rotations were measured in PD rats. Phosphorylated levels of GluR1 at Serine-845 (pGluR1S845) levels were determined by western blot. Arc and Penk levels were measured by real-time polymerase chain reaction (PCR). Results: We found that both 2 μg and 5 μg KN-93 treatment lowered AIMs scores in levodopa priming PD rats without affecting the antiparkinsonian effect of levodopa. In agreement with behavioral analysis, KN-93 treatment (2 μg) reduced pGluR1S845 levels in PD rats. Moreover, KN-93 treatment (2 μg) reduced the expression of Gad1 and Nur77 in PD rats. Conclusion: These data indicated that intrastriatal injections of KN-93 were beneficial in reducing the expression of LID by lowering the expression of pGluR1S845 via suppressing the activation of CaMKII in PD rats. Decreased expression of pGluR1S845 further reduced the expression of Gad1 and Nur77 in PD rats.[5] Taken together, we found that intrastriatal administration of KN-93 (2 μg or 5 μg) reduced the expression of LID in levodopa primed PD rats. In addition, KN-93 treatment reduced pGluR1S845 levels and the expression of Gad1 and Nur77. We assume that KN-93 can ameliorate LID expression by reducing the expression of Gad1 and Nur77 which subsequently lowers the levels of pGluR1S845 in PD rats.[5] ackground: Curcumin possesses many pharmacological properties including anti-inflammatory effects. Although prior studies indicate that curcumin has beneficial effects for diabetic retinopathy, the mechanism of action is not known. To address this issue, we investigated the effect of curcumin against diabetes-induced retinal vascular damage and its mechanism of action by using cultured retinal Müller cells stimulated with high glucose. Methods: We studied the effects of curcumin in vivo in the retinas of rats rendered diabetic by streptozotocin and in vitro in Müller cells stimulated with high glucose. We administered curcumin, or KN93, an inhibitor of calcium/calmodulin dependent protein kinase II (CaMKII), or saline vehicle to experimental animals on a daily basis for 12 weeks. Primary cultures of rat Müller cells were incubated with normal glucose or high glucose, with or without curcumin, KN93, or pyrrolidine dithiocarbamate (PDTC), an inhibitor of the transcription protein nuclear factor κB (NF-κB). We examined mRNA and protein levels of vascular endothelial growth factor (VEGF), inducible nitric oxide synthase (iNOS) and intercellular adhesion molecule-1 (ICAM-1) by real-time RT-PCR and Western blotting, respectively. Retinal levels of CaMKII and NF-κB were examined by Western blotting. Vascular leakage was evaluated using Evans blue. Results: Curcumin and KN93 significantly inhibited the activation of CaMKII/NF-κB signaling induced by diabetes or elevated glucose, and subsequently decreased the expression of VEGF, iNOS and ICAM-1. These changes were associated with a decrease of diabetes-induced retinal vascular leakage. Conclusion: Curcumin protects the diabetic rat retina against early retinal vascular damage, by inhibition of CaMKII activity. Curcumin is currently used to treat a number of clinical conditions, and may prove beneficial for the management of diabetic retinopathy.[4] We reported that one of the isoquinolinesulfonamide derivatives, KN-62, is a potent and specific inhibitor of Ca2+/calmodulin-dependent protein kinase II (CaMKII) (Tokumitsu, H., Chijiwa, T., Hagiwara, M., Mizutani, A., Terasawa, M. and Hidaka, H. (1990) J. Biol. Chem. 265, 4315-4320). We have now investigated the inhibitory property of a newly synthesized methoxybenzenesulfonamide, KN-93, on CaMKII activity in situ and in vitro. KN-93 elicited potent inhibitory effects on CaMKII phosphorylating activity with an inhibition constant of 0.37 microM but this compound had no significant effects on the catalytic activity of cAMP-dependent protein kinase, Ca2+/phospholipid dependent protein kinase, myosin light chain kinase and Ca(2+)-phosphodiesterase. KN-93 also inhibited the autophosphorylation of both the alpha- and beta-subunits of CaMKII. Kinetic analysis indicated that KN-93 inhibits CaMKII, in a competitive fashion against calmodulin. To evaluate the regulatory role of CaMKII on catecholamine metabolism, we examined the effect of KN-93 on dopamine (DA) levels in PC12h cells. The DA levels decreased in the presence of KN-93. Further, the tyrosine hydroxylase (TH) phosphorylation induced by KCl or acetylcholine was significantly suppressed by KN-93 in PC12h cells while events induced by forskolin or 8-Br-cAMP were not affected. These results suggest that KN-93 inhibits DA formation by modulating the reaction rate of TH to reduce the Ca(2+)-mediated phosphorylation levels of the TH molecule.[6] |
| Molecular Formula |
C26H32CLN2O8PS
|
|
|---|---|---|
| Molecular Weight |
599.03
|
|
| Exact Mass |
598.131
|
|
| Elemental Analysis |
C, 52.13; H, 5.38; Cl, 5.92; N, 4.68; O, 21.37; P, 5.17; S, 5.35
|
|
| CAS # |
1188890-41-6
|
|
| Related CAS # |
KN-93 hydrochloride;1956426-56-4;KN-93 phosphate;1913269-12-1; 1188890-41-6 (phosphate); 139298-40-1
|
|
| PubChem CID |
16760530
|
|
| Appearance |
White to off-white solid powder
|
|
| LogP |
4.833
|
|
| Hydrogen Bond Donor Count |
4
|
|
| Hydrogen Bond Acceptor Count |
10
|
|
| Rotatable Bond Count |
11
|
|
| Heavy Atom Count |
39
|
|
| Complexity |
763
|
|
| Defined Atom Stereocenter Count |
0
|
|
| SMILES |
CN(C/C=C/C1=CC=C(C=C1)Cl)CC2=CC=CC=C2N(CCO)S(=O)(=O)C3=CC=C(C=C3)OC.OP(=O)(O)O
|
|
| InChi Key |
NNKJTPOXLIILMB-IPZCTEOASA-N
|
|
| InChi Code |
InChI=1S/C26H29ClN2O4S.H3O4P/c1-28(17-5-6-21-9-11-23(27)12-10-21)20-22-7-3-4-8-26(22)29(18-19-30)34(31,32)25-15-13-24(33-2)14-16-25;1-5(2,3)4/h3-16,30H,17-20H2,1-2H3;(H3,1,2,3,4)/b6-5+;
|
|
| Chemical Name |
(E)-N-(2-(((3-(4-chlorophenyl)allyl)(methyl)amino)methyl)phenyl)-N-(2-hydroxyethyl)-4-methoxybenzenesulfonamide phosphate
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples.
Injection Formulations
Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline)(e.g. IP/IV/IM/SC) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). View More
Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] Oral Formulations
Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). View More
Oral Formulation 3: Dissolved in PEG400 (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6694 mL | 8.3468 mL | 16.6937 mL | |
| 5 mM | 0.3339 mL | 1.6694 mL | 3.3387 mL | |
| 10 mM | 0.1669 mL | 0.8347 mL | 1.6694 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
 |