| Size | Price | Stock | Qty |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
Purity: ≥98%
| Targets |
Metabolic product of cystine
|
|---|---|
| ln Vitro |
Thiotaurine, a metabolic product of cystine, contains a sulfane sulfur atom that can be released as H(2)S, a gaseous molecule with a regulatory activity on inflammatory responses. The influence of thiotaurine on human leukocyte spontaneous apoptosis has been evaluated by measuring caspase-3 activity in human neutrophils. Addition of 100 μM thiotaurine induced a 55% inhibition of caspase-3 activity similar to that exerted by 100 μM H(2)S. Interestingly, in the presence of 1 mM GSH, an increase of the inhibition of apoptosis by thiotaurine has been observed. These results indicate that the bioactivity of thiotaurine can be modulated by GSH, which promotes the reductive breakdown of the thiosulfonate generating H(2)S and hypotaurine. As thiotaurine is able to incorporate reversibly reduced sulfur, it is suggested that the biosynthesis of this thiosulfonate could be a means to transport and store H(2)S [1].
|
| ln Vivo |
Thiotaurine is an effective antioxidant agent as demonstrated by its ability to counteract the damage caused by pro-oxidants in the rat [2].
|
| Enzyme Assay |
Thiotaurine is a thiosulfonate compound bearing a sulfane sulfur atom metabolically generated in body fluids and tissues. Thiotaurine constitutes an interconnection molecule between aerobic and anaerobic pathways of cysteine metabolism. Thiotaurine formed as a result of the reaction between hypotaurine and sulfide may be converted back to H2S and hypotaurine. Thus, thiotaurine may be considered as a safe, non-toxic storage form of H2S and an important key intermediate in the biochemical routes of transport, storage and release of sulfide. Sulfane sulfur-containing compounds efficiently regulate the activity of enzymes and exhibit antioxidative properties. Interestingly, thiotaurine influences inflammatory processes modulating functional responses of human neutrophils and exhibits a protective effect against oxidative damage [2].
|
| Cell Assay |
Influence of Thiotaurine on Human Neutrophil Spontaneous Apoptosis[1]
Spontaneous apoptosis was evaluated by measuring caspase-3 activity in lysates of neutrophils (5 × 106 cells/mL) that were preincubated at 37°C for 3.5 h. When the preincubation step was performed in the presence of thiotaurine (TTAU), a concentration-dependent decrease of caspase-3 activity was observed (Fig. 19.1). As thiotaurine contains a sulfane sulfur atom that can be released as H2S, the influence of NaHS on caspase-3 activity has been also evaluated. With 100 μM thiotaurine the reduction of caspase-3 activity was 55 ± 3%, similar to that exhibited by 100 μM NaHS (57 ± 3%). Control experiments (not shown) indicated that neither TTAU, nor NaHS, at concentrations ranging from 0.01 to 0.2 mM, affected the activity of recombinant caspase-3. Effect of Glutathione on Thiotaurine-Induced Inhibition of Caspase-3 Activity[1] It is reported that glutathione (GSH) regulates neutrophil apoptosis by affecting caspase-3 activity (O’Neill et al. 2000). This effect has been attributed to its antioxidant activity (Wedi et al. 1999). To gain insights into the mechanism of inhibition by TTAU, the inhibitory effect of this thiosulfonate on caspase-3 activity has been compared with that of GSH (Fig. 19.2). |
| References |
[1]. Adv Exp Med Biol. 2013:775:227-36. doi: 10.1007/978-1-4614-6130-2_19.
[2]. Adv Exp Med Biol. 2019:1155:755-771. doi: 10.1007/978-981-13-8023-5_66. |
| Additional Infomation |
These results indicate that the thiosulfonate, thiotaurine, may exert regulatory effects on inflammation influencing lifespan of human neutrophils. Mature circulating neutrophils are constitutively committed to apoptosis. During inflammatory response, survival of neutrophils recruited into the inflamed area is significantly prolonged. Increased survival in the inflamed tissue permits neutrophils to fulfill their effector functions most efficiently. On the other hand, macrophage-mediated elimination of apoptotic neutrophils from the inflamed area has been recognized as a crucial mechanism for promoting resolution of inflammation (Savill and Fadok 2000; Simon 2003). It is recognized that the production of reactive oxygen species by activated cells accelerate the apoptosis and that superoxide release is required for spontaneous apoptosis (Ottonello et al. 2002; Scheel-Toellner et al. 2004). Moreover, the spontaneous and FAS-mediated apoptosis are prevented by antioxidants, such as GSH (Wedi et al. 1999). This effect has been ascribed to the ability of GSH to scavenge reactive oxygen species (Watson et al. 1997). It has been also shown that thiotaurine is highly effective in counteracting the damaging effect of oxidants (Acharya and Lau-Cam 2012). Thus, it is possible that the delay of spontaneous apoptosis of human neutrophils by thiotaurine may be related to its antioxidant activity. On the other hand, our results show that the inhibitory effect of thiotaurine on caspase-3 activity was higher than that of GSH. Moreover thiotaurine, in the presence of GSH, is more effective in influencing neutrophil apoptosis. These findings suggest that alternative or additional mechanisms of inhibition can be involved. It is well-known that GSH can act as a catalyst of the reductive breakdown of thiotaurine with generation of hypotaurine and H2S (Chauncey and Westley 1983). Accordingly, we found that human neutrophils generate H2S from thiotaurine with GSH as a necessary reductant in the reaction. It has been previously reported that H2S promotes the short-term survival of neutrophils by inhibition of caspase-3 cleavage (Rinaldi et al. 2006). Our results confirm the effect of H2S on prolonging the survival of neutrophils. Hence, it is likely that the sulfane sulfur of thiotaurine released as H2S in the presence of GSH, may contribute to the observed effect on neutrophil survival.[1]
The biological relevance of thiotaurine in mammalian is still a challenge to biochemical research. Biological roles have been sporadically reported (Costa et al. 1990; Baskin et al. 2000). On the contrary, in some marine organisms a key role for thiotaurine in the transport of sulfur has been strongly demonstrated (Pruski et al. 2001; Pruski and Fiala-Médioni 2003). Morevover, the metabolic origin of thiotaurine in mammalians is subject to debate, as is its fate. One pathway for thiotaurine metabolism is via transulfuration reactions with hypotaurine being the main intermediate (Cavallini et al. 1961; De Marco and Tentori 1961). These reactions can be spontaneous or catalyzed by sulfur transferases (De Marco et al. 1961; Chauncey and Westley 1983). Our experiments show that hypotaurine is the main metabolite of thiotaurine with a 1:1 stoichiometry, suggesting a role of thiotaurine as a biochemical intermediate in the transport, storage, and release of sulfide also in mammalians. This hypothesis is further supported by the fact that hypotaurine, present in leukocytes at millimolar concentration (Learn et al. 1990), can readily incorporate H2S formed during inflammation with production of thiotaurine (De Marco and Tentori 1961).[1] Since thiotaurine as well as hypotaurine, taurine, and H2S can modulate leukocyte functional responses, it would be worthy to investigate the metabolic and functional interplay between these sulfur compounds at inflammatory sites.[1] |
| Molecular Weight |
141.21248
|
|---|---|
| Exact Mass |
140.992
|
| CAS # |
2937-54-4
|
| Related CAS # |
31999-89-0 (mono-hydrochloride salt);2937-54-4 (Parent)
|
| PubChem CID |
6858023
|
| Appearance |
Solid
|
| Density |
1.541g/cm3
|
| Boiling Point |
324.6ºC at 760mmHg
|
| Flash Point |
150.1ºC
|
| Vapour Pressure |
4.94E-05mmHg at 25°C
|
| Index of Refraction |
1.622
|
| LogP |
0.894
|
| Hydrogen Bond Donor Count |
2
|
| Hydrogen Bond Acceptor Count |
4
|
| Rotatable Bond Count |
2
|
| Heavy Atom Count |
7
|
| Complexity |
137
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
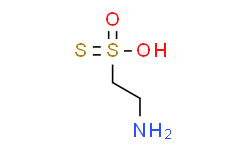
NCCS(=S)(O)=O
|
| InChi Key |
SHWIJIJNPFXOFS-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C2H7NO2S2/c3-1-2-7(4,5)6/h1-3H2,(H,4,5,6)
|
| Chemical Name |
2-hydroxysulfonothioylethanamine
|
| Synonyms |
Thiotaurine; 2-hydroxysulfonothioylethanamine; 2-Aminoethanethiosulfonic acid; NQZ2D7AO62; Thiotaurine; 2937-54-4; 2-hydroxysulfonothioylethanamine; 2-Aminoethanethiosulfonic acid; NQZ2D7AO62; Sodium 2-aminosulphonothioacetate; EINECS 250-888-0; 31999-89-0;
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples.
Injection Formulations
Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline)(e.g. IP/IV/IM/SC) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). View More
Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] Oral Formulations
Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). View More
Oral Formulation 3: Dissolved in PEG400 (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 7.0817 mL | 35.4083 mL | 70.8165 mL | |
| 5 mM | 1.4163 mL | 7.0817 mL | 14.1633 mL | |
| 10 mM | 0.7082 mL | 3.5408 mL | 7.0817 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.