| Size | Price | Stock | Qty |
|---|---|---|---|
| 500mg |
|
| Targets |
Microbial Metabolite; Endogenous Metabolite; Flavoring Agents; Alters several flavor and/or taste characteristics; Food additives; Fragrance Ingredients; Cosmetics -> Buffering; Environmental transformation -> Pesticide transformation products (metabolite, successor)
|
|---|---|
| ln Vitro |
Succinic acid is considered as an important platform chemical. Succinic acid fermentation with Actinobacillus succinogenes strain BE-1 was optimized by central composite design (CCD) using a response surface methodology (RSM). The optimized production of succinic acid was predicted and the interactive effects between glucose, yeast extract, and magnesium carbonate were investigated. As a result, a model for predicting the concentration of succinic acid production was developed. The accuracy of the model was confirmed by the analysis of variance (ANOVA), and the validity was further proved by verification experiments showing that percentage errors between actual and predicted values varied from 3.02% to 6.38%. In addition, it was observed that the interactive effect between yeast extract and magnesium carbonate was statistically significant. In conclusion, RSM is an effective and useful method for optimizing the medium components and investigating the interactive effects, and can provide valuable information for succinic acid scale-up fermentation using A. succinogenes strain BE-1[1].
|
| ln Vivo |
Male mice that are given succinic acid (3, 6 mg/kg; oral) have higher percentages of entrances into the open arm and longer durations there [3]. Food intake was considerably enhanced by succinic acid (3, 6, 12 mg/kg; ip) between 5 and 40 minutes after delivery. Rectal temperature was taken, and 1.5 mg/kg of succinic acid was found to prevent stress-induced hyperthermia [3].
|
| Animal Protocol |
The putative anxiolytic activity of succinic acid was examined in male mice by using a number of experimental paradigms of anxiety and compared with that of the known anxiolytic compound diazepam. Use of the elevated plus-maze test revealed that diazepam (1.0, 2.0 and 4.0 mg/kg, PO) or succinic acid (3.0 or 6.0 mg/kg, PO) increased the percentage of entries into open arms and of time spent on open arms. In novel food consumption test, succinic acid (3.0, 6.0, and 12.0 mg/kg, IP) caused significant increases in food intake during 5 min when compared with the vehicle. In the stress-induced hyperthermia test, 40 min after drug administration rectal temperature was measured, succinic acid at dose of 1.5 mg/kg, inhibited stress-induced hyperthermia. Thus, these findings indicated that, in contrast with diazepam, succinic acid exhibits anxiolytic-like effect.[3]
|
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
Succinic acid occurs normally in human urine (1.9-8.8 mg/L). Metabolism / Metabolites Succinic acid is a normal intermediary metabolite and a constituent of the citric acid cycle. It is readily metabolized when administered to animals, but may be partly excreted unchanged in the urine if large doses are fed. Succinic acid can be converted into fumaric acid by oxidation via succinate dehydrogenase. Agrochemical Transformations Butanedioic acid is a known environmental transformation product of Sulcotrione. Succinic acid is a known environmental transformation product of Linuron. |
| Toxicity/Toxicokinetics |
mouse LD50 intravenous 4500 mg/kg Merck Index; an Encyclopedia of Chemicals, Drugs, and Biologicals, 11th ed., Rahway, NJ 07065, Merck & Co., Inc. 1989, 11(1368), 1989
Toxicity Summary Succinate can inhibit the activities of α-KG–dependent oxygenases (KDMs) and the TET family of 5-methlycytosine (5mC) hydroxylases. Succinate also mediates allosteric inhibition of hypoxia inducible factor (HIF) prolyl hydroxylases (PHDs). Inhibition of HIF PHDs leads to activation of HIF-mediated pseudohypoxic response, whereas inhibition of KDMs and TET family of 5mC hydroxylases causes epigenetic alterations that ultimately cause cancer. Succination of KEAP1 in FH deficiency results in the constitutive activation of the antioxidant defense pathway mediated by NRF2, conferring a reductive milieu that promotes cell proliferation. Succination of the Krebs cycle enzyme Aco2 impairs aconitase activity in Fh1-deficient MEFs. Succination also causes irreversible inactivation of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Health Effects At acute doses or exposures succinic acid is a skin irritant. Chronically high doses of succinate can lead to succinylation or succination of a variety of enzymes. Partial succinate dehydrogenase deficiency (15% to 50% of normal reference enzyme activity) in skeletal muscle leads to elevated succinate levels and causes mitochondrial myopathy with various symptoms, for example, brain involvement, cardiomyopathy, and/or exercise intolerance. Exposure Routes Eye contact, Inhalation, Ingestion. Symptoms Acute Exposure: the clinical signs of acute toxicity are weakness and diarrhea. Adverse Effects Neurotoxin - Other CNS neurotoxin View More
Toxicity Data
Treatment EYES: irrigate opened eyes for several minutes under running water. INGESTION: do not induce vomiting. Rinse mouth with water (never give anything by mouth to an unconscious person). Seek immediate medical advice. SKIN: should be treated immediately by rinsing the affected parts in cold running water for at least 15 minutes, followed by thorough washing with soap and water. If necessary, the person should shower and change contaminated clothing and shoes, and then must seek medical attention. INHALATION: supply fresh air. If required provide artificial respiration. Human Toxicity Excerpts /OTHER TOXICITY INFORMATION/ Primary irritant effects are present with a number of ... /aliphatic dicarboxylic/ acids, particularly in concentrated solution or as dusts- sensitization is rare. /Aliphatic dicarboxylic acids/ International Labour Office. Encyclopedia of Occupational Health and Safety. Volumes I and II. New York: McGraw-Hill Book Co., 1971., p. 30 Non-Human Toxicity Excerpts /LABORATORY ANIMALS: Acute Exposure/ Succinic acid is slight skin irritant and a strong eye irritant in rats. Application of 750 ug of succinic acid as a 15% solution produced severe damage in rabbit eyes. The clinical signs of acute toxicity in rats are weakness and diarrhea. /LABORATORY ANIMALS: Acute Exposure/ Large iv doses of sodium succinate produced vomiting and diarrhea in cats... . /LABORATORY ANIMALS: Subchronic or Prechronic Exposure/ Rats/Fischer (F344) males and females,10 per group /were exposed for/ 13 weeks ad libitum /to/ 0, 0.3, 0.6, 1.25, 2.5, 5, 10% monosodium succinate, purity 100.2%. ...Severe suppression of body weight gain occurred in rats in the 10% group, and all of the rats in this group died during the first 4 weeks of the experiment. However, in the other dose groups all of the rats survived to the end of the experiment. Suppression of body weight gain was observed at >/=2.5%. The volume of drinking water consumed was very small in the highest dose groups, although it was larger in the 5% group than in the other groups. No specific dose-related changes were observed in any parameters in the hematological and biochemical investigations. Rats that died during the experiment were severely emaciated. However, no toxic lesions caused by the test substance were found in any organs of these rats histopathologically, although atrophy of the organs was observed. No specific lesions were observed histologically in any of the other test groups. On the basis of body weight depression, the maximum tolerated dose of monosodium succinate was determined to be approximately 2-2.5% when given in the drinking water. Non-Human Toxicity Values LD50 Rat oral 2260 mg/kg |
| References |
|
| Additional Infomation |
Sodium succinate (anhydrous) is a sodium salt that is the disodium salt of succinic acid. The hexahydrate form is used as an ingredient of topical preparations for the treatment of cataract. It contains a succinate(2-).
See also: Succinic Acid (has active moiety). |
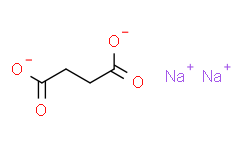
| Molecular Formula |
C4H4NA2O4
|
|---|---|
| Molecular Weight |
162.05
|
| Exact Mass |
161.99
|
| Elemental Analysis |
C, 29.65; H, 2.49; Na, 28.37; O, 39.49
|
| CAS # |
150-90-3
|
| PubChem CID |
9020
|
| Appearance |
White to off-white solid powder
|
| Flash Point |
110.9ºC
|
| Vapour Pressure |
0.0165mmHg at 25°C
|
| Hydrogen Bond Donor Count |
0
|
| Hydrogen Bond Acceptor Count |
4
|
| Rotatable Bond Count |
1
|
| Heavy Atom Count |
10
|
| Complexity |
81.6
|
| Defined Atom Stereocenter Count |
0
|
| InChi Key |
ZDQYSKICYIVCPN-UHFFFAOYSA-L
|
| InChi Code |
InChI=1S/C4H6O4.2Na/c5-3(6)1-2-4(7)8;;/h1-2H2,(H,5,6)(H,7,8);;/q;2*+1/p-2
|
| Chemical Name |
disodium;butanedioate
|
| Synonyms |
CCRIS-3700; Disodium succinate; 150-90-3; SODIUM SUCCINATE; Disodium butanedioate; Succinic acid disodium salt; Butanedioic acid, disodium salt; FEMA No. 3277; Sodium succinate dibasic; CCRIS 3700; Butanedioic acid, disodium salt
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples.
Injection Formulations
Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline)(e.g. IP/IV/IM/SC) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). View More
Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] Oral Formulations
Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). View More
Oral Formulation 3: Dissolved in PEG400 (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 6.1709 mL | 30.8547 mL | 61.7093 mL | |
| 5 mM | 1.2342 mL | 6.1709 mL | 12.3419 mL | |
| 10 mM | 0.6171 mL | 3.0855 mL | 6.1709 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.