| Size | Price | Stock | Qty |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
Caffeine is a naturally occurring methylxanthine found in some beverages and also used as a pharmacological agent. Caffeine's most notable pharmacological effect is as a central nervous system stimulant, increasing alertness and producing agitation. It also relaxes smooth muscle, stimulates cardiac muscle, stimulates diuresis, and appears to be useful in the treatment of some types of headache. Several cellular actions of caffeine have been observed, but it is not entirely clear how each contributes to its pharmacological profile. Among the most important are inhibition of cyclic nucleotide phosphodiesterases, antagonism of adenosine receptors, and modulation of intracellular calcium handling.
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
Caffeine is rapidly absorbed after oral or parenteral administration, reaching peak plasma concentration within 30 minutes to 2 hours after administration. After oral administration, onset of action takes place within 45 to 1 hour. Food may delay caffeine absorption. The peak plasma level for caffeine ranges from 6-10mg/L. The absolute bioavailability is unavailable in neonates, but reaches about 100% in adults. The major metabolites of caffeine can be found excreted in the urine. About 0.5% to 2% of a caffeine dose is found excreted in urine, as it because it is heavily absorbed in the renal tubules. Caffeine has the ability to rapidly cross the blood-brain barrier. It is water and fat soluble and distributes throughout the body. Caffeine concentrations in the cerebrospinal fluid of preterm newborns are similar to the concentrations found in the plasma. The mean volume of distribution of caffeine in infants is 0.8-0.9 L/kg and 0.6 L/kg in the adult population. The clearance of caffeine varies, but on average, is about 0.078 L/kg/h (1.3 mL/min/kg). World-wide, many fetuses and infants are exposed to methylxanthines via maternal consumption of coffee and other beverages containing these substances. Methylxanthines (caffeine, theophylline and aminophylline) are also commonly used as a medication for apnea of prematurity. ... Methylxanthines readily passes the placenta barrier and enters all tissues and thus may affect the fetus/newborn at any time during pregnancy or postnatal life, given that the effector systems are mature. ... Caffeine and citrated caffeine are well absorbed following oral administration. Absorption of caffeine following oral administration may be more rapid than that following IM injection of caffeine and sodium benzoate. Absorption following rectal administration of caffeine in suppositories may be slow and erratic. ... Following oral administration of 100 mg of caffeine (as coffee), peak plasma concentrations of about 1.5-1.8 ug/mL are reached after 50-75 minutes. After oral administration of 10 mg caffeine base/kg to preterm neonates, the peak plasma concentration for caffeine ranged from 6-10 mg/L and the mean time to reach peak concentration /Tmax/ ranged from 30 minutes to 2 hours. The /Tmax/ was not affected by formula feeding. Caffeine is rapidly distributed into body tissues, readily crossing the placenta and blood-brain barrier. Caffeine concentration in the CSF fluid of preterm neonates approximates the plasma concentration. The mean volume of distribution of caffeine in infants (0.8-0.9 L/kg) is slightly higher than that in adults (0.6 L/kg). ... Caffeine has been shown to distribute into milk in a milk-to-serum concentration ratio of 0.5-0.76. For more Absorption, Distribution and Excretion (Complete) data for CAFFEINE (11 total), please visit the HSDB record page. Metabolism / Metabolites Caffeine metabolism occurs mainly in the liver via the cytochrome CYP1A2 enzyme. The products of caffeine metabolism include paraxanthine, theobromine, and theophylline. The first step of caffeine metabolism is demethylation, yielding paraxanthine (a major metabolite), followed by theobromine, and theophylline, which are both minor metabolites. They are then excreted in urine as urates after additional metabolism. The enzymes xanthine oxidase and N-acetyltransferase 2 (NAT2) also participate in the metabolism of caffeine. Caffeine is metabolized by the cytochrome P-450 (CYP) enzyme system, principally by isoenzyme 1A2. Therefore, caffeine has the potential to interact with drugs that are metabolized by CYP1A2 or with drugs that induce or inhibit this isoenzyme. In adults, the drug is rapidly metabolized in the liver to 1-methyluric acid, 1-methylxanthine, and 7-methylxanthine. Interconversion between caffeine and theophylline has been reported in preterm neonates... In-vivo and in-vitro experiments showed a progressive increase in the activity of the hepatic microsomal enzymes that metabolize caffeine during neonatal development. In beagle puppies, change in caffeine clearance was determined by the rate of maturation of caffeine-7-demethylase. Caffeine is eliminated in animals by biotransformation in the liver to dimethylxanthines, dimethyl- and monomethyluric acids and uracil derivatives; important quantitative differences have been demonstrated in the formation and elimination of metabolites in rats, mice and Chinese hamsters. These differences are even more important in monkeys, where caffeine is almost completely metabolized to theophylline. ... Some species-dependent metabolites have been identified. Trimethylallantoin was first reported in rats. A ... derivative of paraxanthine was found in mice and identified as the 3-beta-D-glucuronide of paraxanthine. Methylated ureas and sulfur-containing derivatives found in urine in trace amounts are produced by the intestinal flora. In contrast, the acetylated uracil derivative, 5-acetylamino-6-formylamino-3-methyluracil, one of the most important caffeine metabolites in humans, has not been identified in rodents or other animal species. Other uracil derivatives produced from caffeine, theobromine and paraxanthine in rats were found in human urine. In rats, the hepatic demethylation of caffeine shows an age-related decline, resulting in a greatly increased elimination half-time in older adult rats. Caffeine metabolism is qualitatively relatively similar in animals and humans ... . The main metabolic pathways are: demethylation and hydroxylation of the 8-position leading to the formation of the respective uracil and uric acid derivatives. There are, however, some quantitative differences in the metabolic profile. Humans are characterized by the importance of 3-methyl demethylation leading to the formation of paraxanthine and especially metabolites thereof through subsequent metabolic steps. The main urinary metabolites in humans are 1-methyluric acid, 1-methylxanthine, 5-acetylamino-6-formylamino-3- methyluracil (not found in rats and mice), 1,7-dimethyluric acid and paraxanthin. In rats and mice, the metabolism of caffeine is predominantly via theobromine and theophylline. The main urinary metabolites are 1,3-dimethyluracil, paraxanthine, trimethyluric acid, theophylline, and theobromine. Caffeine metabolism decreases during pregnancy, resulting in higher serum concentrations. Caffeine has known human metabolites that include Theobromine, theophylline, 1,3,7-Trimethyluric acid, and paraxanthine. Hepatic cytochrome P450 1A2 (CYP 1A2) is involved in caffeine biotransformation. About 80% of a dose of caffeine is metabolized to paraxanthine (1,7-dimethylxanthine), 10% to theobromine (3,7-dimethylxanthine), and 4% to theophylline (1,3-dimethylxanthine). Route of Elimination: In young infants, the elimination of caffeine is much slower than that in adults due to immature hepatic and/or renal function. Half Life: 3 to 7 hours in adults, 65 to 130 hours in neonates Biological Half-Life In an average-sized adult or child above the age of 9, the half-life of caffeine is approximately 5 hours. Various characteristics and conditions can alter caffeine half-life. It can be reduced by up to 50% in smokers. Pregnant women show an increased half-life of 15 hours or higher, especially in the third trimester. The half-life in newborns is prolonged to about 8 hours at full-term and 100 hours in premature infants, likely due to reduced ability to metabolize it. Liver disease or drugs that inhibit CYP1A2 can increase caffeine half-life. Elimination 1/2 life in adults = 2.5-4.5 hours; [Reference #1] Caffeine has a plasma half-life (t1/2) of 3 to 5 hours in adults. In one study, when administered to pregnant women prior to delivery, caffeine had a prolonged mean half-life of 80 hours in neonates after delivery. Mean half-life /T 1/2/ and fraction excreted unchanged in urine of caffeine in infants have been shown to be inversely related to gestational/postconceptual age. In neonates, the /T 1/2/ is approximately 3-4 days... The half-time for caffeine is 0.7-1.0 hr in rats and mice, 1-1.6 hr in rabbits, 3-5 hr in monkeys, 4-4.3 hr in dogs and 11-12 hr in baboons. /The authors/ studied 17 preterm infants receiving caffeine, and measured their plasma levels of caffeine and the theophylline metabolite by high-pressure liquid chromatography. The half-life was calculated by computer analysis using the least-square method. The mean gestational age of our patients was 29.7 +/- 1.9 weeks (mean +/- SD) and they were studied at 20.7 +/- 6.6 days (mean +/- SD) postnatal age. The caffeine half-life was 52.03 +/- 23.87 hr (means +/- SD) and the theophylline half-life was 77.04 +/- 65.01 hr (mean +/- SD). |
|---|---|
| Toxicity/Toxicokinetics |
Toxicity Summary
Caffeine stimulates medullary, vagal, vasomotor, and respiratory centers, promoting bradycardia, vasoconstriction, and increased respiratory rate. This action was previously believed to be due primarily to increased intracellular cyclic 3′,5′-adenosine monophosphate (cyclic AMP) following inhibition of phosphodiesterase, the enzyme that degrades cyclic AMP. It is now thought that xanthines such as caffeine act as antagonists at adenosine-receptors within the plasma membrane of virtually every cell. As adenosine acts as an autocoid, inhibiting the release of neurotransmitters from presynaptic sites but augmenting the actions of norepinephrine or angiotensin, antagonism of adenosine receptors promotes neurotransmitter release. This explains the stimulatory effects of caffeine. Blockade of the adenosine A1 receptor in the heart leads to the accelerated, pronounced "pounding" of the heart upon caffeine intake. Toxicity Data LD50: 127 mg/kg (Oral, Mouse) (A308) Interactions When caffeine and disulfiram are administered concomitantly...the total blood clearance of caffeine is substantially decreased and its elimination half-life is increased. ... Cocaine and caffeine's independent effects on cardiodynamics are documented but to our knowledge combined effects of both on complete cardiovascular hemodynamics remains to be examined. Eighteen dogs were instrumented to pass cardiac catheters into right and left heart. The experiments were performed after they recovered from the effects of anesthesia. In phase I (30 experiments on 8 dogs), the doses were established by dose-response curve. In phases II and III, another 10 dogs were subjected to 28 experiments. They were given i.v. cocaine followed by caffeine and vice versa to study their effects on hemodynamics and coronary flow reserve. Phase 1: The doses of cocaine (2 mg/kg) and caffeine (5 mg/kg) were established. Phase II: Cocaine increased heart rate, blood pressure and dP/dt but coronary flow reserve (CFR) decreased significantly. Caffeine administered after cocaine attenuated these effects (dP/dt decreased to 4910 + or -104 from 5066 + or - 110 mm Hg s; p The effects of the widely consumed drugs caffeine and phenylpropanolamine are mediated through activation of the central and sympathetic nervous systems. Severe, life-threatening, and occasionally fatal hypertensive reactions have been reported after their combined use. This study examined the possible pharmacokinetic interaction of phenylpropanolamine and caffeine. Sixteen normal subjects received combinations of caffeine, phenylpropanolamine, and placebo. In subjects receiving 400 mg caffeine plus 75 mg phenylpropanolamine, the mean (+/- SEM) peak plasma caffeine concentration of 8.0 +/- 2.2 micrograms/mL was significantly greater than after 400 mg caffeine alone (2.1 +/- 0.3 micrograms/mL; t[24] = 2.4; p less than 0.01). Physical side effects were more frequent after the phenylpropanolamine-caffeine combination than after either drug alone or after placebo. Greater increases in both systolic and diastolic blood pressures occurred after the combination than after either drug alone. Because caffeine levels can be increased greatly when certain other drugs are coconsumed, these data indicate that phenylpropanolamine may enhance absorption or inhibit elimination of caffeine and may explain increased side effects reported after their combined use. For more Interactions (Complete) data for CAFFEINE (21 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat ip 260 mg/kg LD50 Rat oral 192 mg/kg LD50 Rat rectal 300 mg/kg LD50 Rat iv 105 mg/kg For more Non-Human Toxicity Values (Complete) data for CAFFEINE (13 total), please visit the HSDB record page. |
| Additional Infomation |
Therapeutic Uses
Central Nervous System Stimulants; Phosphodiesterase Inhibitors; Purinergic P1 Receptor Antagonists Caffeine is used orally as a mild CNS stimulant to aid in staying awake and to restore mental alertness in fatigued patients. Apnea of prematurity. Caffeine citrate is used iv or orally in the short-term (10-12 days) treatment of apnea of prematurity in neonates who are between 28 and less than 33 weeks of gestational age. Caffeine is designated an orphan drug by the US Food and Drug Administration (FDA) for use in apnea in premature neonates. Caffeine is used in combination with ergotamine tartrate to abort vascular headaches such as migraine and cluster headaches (histamine cephalalgia). For more Therapeutic Uses (Complete) data for CAFFEINE (11 total), please visit the HSDB record page. Drug Warnings Because it has been suggested that caffeine may promote gastric ulceration, the drug should be used cautiously in patients with a history of peptic ulcer. Because of its suspected arrhythmogenic potential, it is generally recommended that caffeine be avoided in patients with symptomatic cardiac arrhythmias and/or palpitations and during the first several days to weeks after an acute myocardial infarction Prior to initiation of caffeine citrate therapy, baseline serum concentrations of caffeine should be measured in neonates previously treated with theophylline, since preterm neonates metabolize theophylline to caffeine. Similarly, baseline serum concentrations of caffeine should be measured in infants born to mothers who consumed caffeine prior to delivery since caffeine readily crosses the placenta. Serious toxicity has been reported when serum caffeine concentrations exceed 50 ug/mL. /Caffeine citrate/ In clinical trials reported in the literature, cases of hypoglycemia and hyperglycemia have been reported in patients receiving caffeine; therefore, blood glucose concentration may need to be monitored periodically in neonates receiving caffeine citrate. /Caffeine citrate/ During the placebo-controlled trial of caffeine citrate establishing efficacy in the US for apnea of prematurity, 6 cases of necrotizing enterocolitis developed among the 85 neonates studied, 3 cases of which were fatal. Five of the 6 neonates had been randomized to treatment with or had been exposed to caffeine citrate. Reports in the literature have raised the possibility of an association betwen the use of methylxanthines and the development of necrotizing enterocolitis, although a causal relationship between methylxanthine use and the development of necrotizing enterocolitis have not been established. Therefore, as with all premature neonates, patients being treated with caffeine citrate should be monitored carefully for the development of necrotizing enterocolitis. /Caffeine citrate/ For more Drug Warnings (Complete) data for CAFFEINE (25 total), please visit the HSDB record page. Pharmacodynamics Caffeine stimulates the central nervous system (CNS), heightening alertness, and sometimes causing restlessness and agitation. It relaxes smooth muscle, stimulates the contraction of cardiac muscle, and enhances athletic performance. Caffeine promotes gastric acid secretion and increases gastrointestinal motility. It is often combined in products with analgesics and ergot alkaloids, relieving the symptoms of migraine and other types of headaches. Finally, caffeine acts as a mild diuretic. |
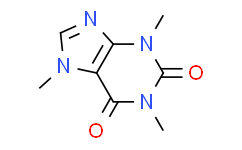
| Molecular Formula |
C8H10N4O2
|
|---|---|
| Molecular Weight |
194.19060087204
|
| Exact Mass |
194.08
|
| CAS # |
58-08-2
|
| PubChem CID |
2519
|
| Appearance |
White, prismatic crystals
Hexagonal prisms White, fleecy masses or long, flexible, silky crystals |
| Melting Point |
460 °F (NTP, 1992)
235-237 236.2 °C 238 °C 235 °C |
| LogP |
-0.1
|
| Hydrogen Bond Donor Count |
0
|
| Hydrogen Bond Acceptor Count |
3
|
| Rotatable Bond Count |
0
|
| Heavy Atom Count |
14
|
| Complexity |
293
|
| Defined Atom Stereocenter Count |
0
|
| InChi Key |
RYYVLZVUVIJVGH-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C8H10N4O2/c1-10-4-9-6-5(10)7(13)12(3)8(14)11(6)2/h4H,1-3H3
|
| Chemical Name |
1,3,7-trimethylpurine-2,6-dione
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
May dissolve in DMSO (in most cases), if not, try other solvents such as H2O, Ethanol, or DMF with a minute amount of products to avoid loss of samples
|
|---|---|
| Solubility (In Vivo) |
Note: Listed below are some common formulations that may be used to formulate products with low water solubility (e.g. < 1 mg/mL), you may test these formulations using a minute amount of products to avoid loss of samples.
Injection Formulations
Injection Formulation 1: DMSO : Tween 80: Saline = 10 : 5 : 85 (i.e. 100 μL DMSO stock solution → 50 μL Tween 80 → 850 μL Saline)(e.g. IP/IV/IM/SC) *Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH ₂ O to obtain a clear solution. Injection Formulation 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (i.e. 100 μL DMSO → 400 μLPEG300 → 50 μL Tween 80 → 450 μL Saline) Injection Formulation 3: DMSO : Corn oil = 10 : 90 (i.e. 100 μL DMSO → 900 μL Corn oil) Example: Take the Injection Formulation 3 (DMSO : Corn oil = 10 : 90) as an example, if 1 mL of 2.5 mg/mL working solution is to be prepared, you can take 100 μL 25 mg/mL DMSO stock solution and add to 900 μL corn oil, mix well to obtain a clear or suspension solution (2.5 mg/mL, ready for use in animals). View More
Injection Formulation 4: DMSO : 20% SBE-β-CD in saline = 10 : 90 [i.e. 100 μL DMSO → 900 μL (20% SBE-β-CD in saline)] Oral Formulations
Oral Formulation 1: Suspend in 0.5% CMC Na (carboxymethylcellulose sodium) Oral Formulation 2: Suspend in 0.5% Carboxymethyl cellulose Example: Take the Oral Formulation 1 (Suspend in 0.5% CMC Na) as an example, if 100 mL of 2.5 mg/mL working solution is to be prepared, you can first prepare 0.5% CMC Na solution by measuring 0.5 g CMC Na and dissolve it in 100 mL ddH2O to obtain a clear solution; then add 250 mg of the product to 100 mL 0.5% CMC Na solution, to make the suspension solution (2.5 mg/mL, ready for use in animals). View More
Oral Formulation 3: Dissolved in PEG400 (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.1496 mL | 25.7480 mL | 51.4960 mL | |
| 5 mM | 1.0299 mL | 5.1496 mL | 10.2992 mL | |
| 10 mM | 0.5150 mL | 2.5748 mL | 5.1496 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
Study to Investigate the Effect of Rocatinlimab (AMG 451) on the Pharmacokinetics of Multiple Cytochrome P450 (CYP450) Substrates in Participants With Moderate to Severe Atopic Dermatitis
CTID: NCT05891119
PhaseEarly Phase 1 Status: Active, not recruiting
Date: 2024-11-07