
| Size | Price | Stock | Qty |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
Purity: ≥98%
SB269970 (SB-269970A) is a novel and potent 5-HT7 receptor antagonist with pKi of 8.3, exhibits >50-fold selectivity against other receptors. SB269970 functions as a 5-HT7 receptor selective antagonist that exhibits at least a 100-fold selectivity against 5-HT7 across a range of receptors and enzymes. Moreover, SB269970 suppresses the 5-CT-stimulated adenylyl cyclase activity in both the guinea-pig hippocampal (pKB of 8.3) and 5-HT7(a)/HEK293 (pA2 of 8.5) membranes.
| Targets |
5-HT7 Receptor ( pKi = 8.3 )
|
||
|---|---|---|---|
| ln Vitro |
|
||
| ln Vivo |
|
||
| Enzyme Assay |
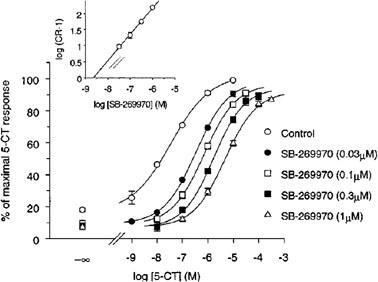
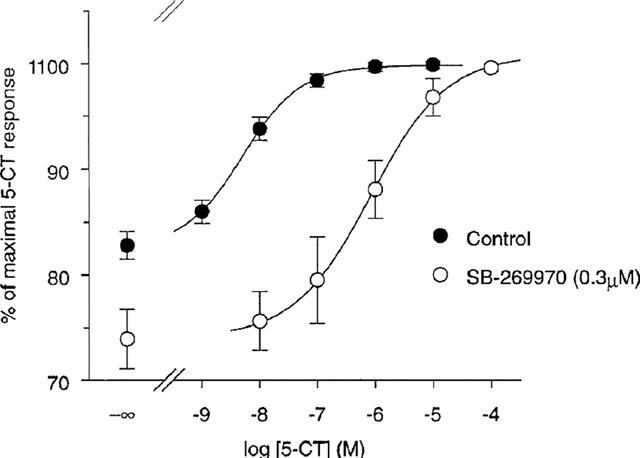
SB269970 an antagonist of the 5-HT7 receptor with pKi of 8.3, exhibits >50-fold selectivity against other receptors. The novel 5-HT(7) receptor antagonist, SB-269970-A, potently displaced [(3)H]-5-CT from human 5-HT(7(a)) (pK(i) 8.9+/-0.1) and 5-HT(7) receptors in guinea-pig cortex (pK(i) 8.3+/-0.2). 5-CT stimulated adenylyl cyclase activity in 5-HT(7(a))/HEK293 membranes (pEC(50) 7.5+/-0.1) and SB-269970-A (0.03 - 1 microM) inhibited the 5-CT concentration-response with no significant alteration in the maximal response. The pA(2) (8.5+/-0.2) for SB-269970-A agreed well with the pK(i) determined from [(3)H]-5-CT binding studies. 5-CT-stimulated adenylyl cyclase activity in guinea-pig hippocampal membranes (pEC(50) of 8.4+/-0.2) was inhibited by SB-269970-A (0.3 microM) with a pK(B) (8.3+/-0.1) in good agreement with its antagonist potency at the human cloned 5-HT(7(a)) receptor and its binding affinity at guinea-pig cortical membranes. 5-HT(7) receptor mRNA was highly expressed in human hypothalamus, amygdala, thalamus, hippocampus and testis. SB-269970-A was CNS penetrant (steady-state brain : blood ratio of ca. 0.83 : 1 in rats) but was rapidly cleared from the blood (CLb=ca. 140 ml min(-1) kg(-1))[1].
|
||
| Cell Assay |
1. The presence of 5-HT(7) receptor mRNA and protein in 5-HT neurons suggests that this receptor may act as a 5-HT autoreceptor. In this study, the effect of the 5-HT(7) receptor antagonist, SB-269970 ((R)-1-[3-hydroxy phenyl)sulfonyl]-2-[2-(4-methyl-1-piperidinyl)ethyl]pyrrolidine), was investigated on 5-HT release in the guinea-pig and rat cortex and the rat dorsal raphe nucleus (DRN), using the techniques of in vitro [(3)H]-5-HT release or fast cyclic voltammetry, respectively. 2. Cortical slices were loaded with [(3)H]-5-HT and release was evoked by electrical stimulation. 5-CT inhibited the evoked release of [(3)H]-5-HT in a concentration-dependent manner. SB-269970 had no significant effect on [(3)H]-5-HT release while the 5-HT(1B) receptor antagonist, SB-224289 significantly potentiated [(3)H]-5-HT release. In addition, SB-269970 was unable to attenuate the 5-CT-induced inhibition of release while SB-224289 produced a rightward shift of the 5-CT response, generating estimated pK(B) values of 7.8 and 7.6 at the guinea-pig and rat terminal 5-HT autoreceptors respectively. 3. Rat DRN slices were electrically stimulated and the evoked 5-HT efflux detected by voltammetric analysis. 8-OH-DPAT inhibited evoked 5-HT efflux and was fully reversed by WAY 100635. SB-269970 had no effect on either 5-HT efflux per se or 8-OH-DPAT-induced inhibition of 5-HT efflux. In addition, 5-CT inhibited 5-HT efflux in a concentration-dependent manner. SB-269970 was unable to attenuate the 5-CT-induced inhibition of 5-HT efflux. 4. In conclusion, we were unable to provide evidence to suggest a 5-HT autoreceptor role for 5-HT(7) receptors. However, investigations with more selective 5-HT(7) receptor agonists are needed to confirm the data reported here.[2]
The novel 5-HT(7) receptor antagonist, SB-269970-A, potently displaced [(3)H]-5-CT from human 5-HT(7(a)) (pK(i) 8.9+/-0.1) and 5-HT(7) receptors in guinea-pig cortex (pK(i) 8.3+/-0.2). 5-CT stimulated adenylyl cyclase activity in 5-HT(7(a))/HEK293 membranes (pEC(50) 7.5+/-0.1) and SB-269970-A (0.03 - 1 microM) inhibited the 5-CT concentration-response with no significant alteration in the maximal response. The pA(2) (8.5+/-0.2) for SB-269970-A agreed well with the pK(i) determined from [(3)H]-5-CT binding studies. 5-CT-stimulated adenylyl cyclase activity in guinea-pig hippocampal membranes (pEC(50) of 8.4+/-0.2) was inhibited by SB-269970-A (0.3 microM) with a pK(B) (8.3+/-0.1) in good agreement with its antagonist potency at the human cloned 5-HT(7(a)) receptor and its binding affinity at guinea-pig cortical membranes. 5-HT(7) receptor mRNA was highly expressed in human hypothalamus, amygdala, thalamus, hippocampus and testis. SB-269970-A was CNS penetrant (steady-state brain : blood ratio of ca. 0.83 : 1 in rats) but was rapidly cleared from the blood (CLb=ca. 140 ml min(-1) kg(-1)). [1] |
||
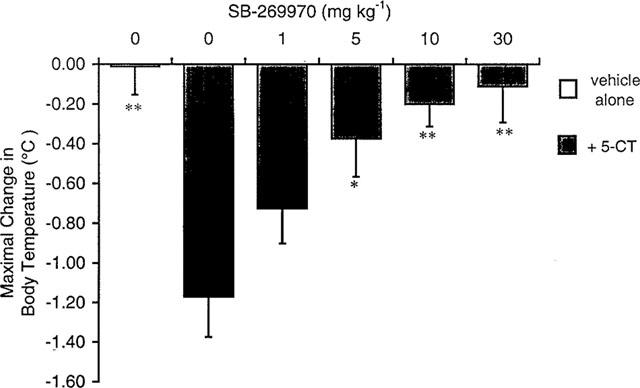
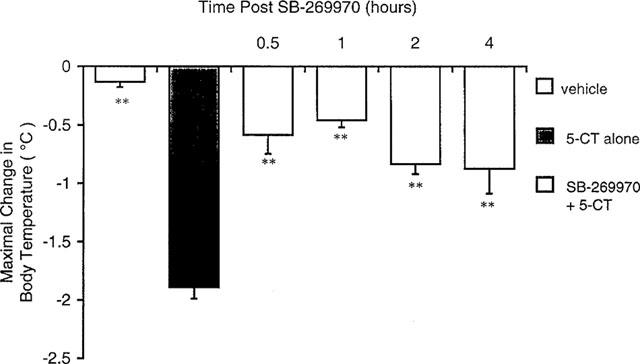
| Animal Protocol |
|
||
| References |
|
||
| Additional Infomation |
3-[[(2R)-2-[2-(4-methyl-1-piperidinyl)ethyl]-1-pyrrolidinyl]sulfonyl]phenol is a sulfonamide.
SB-269970 is an experimental drug developed by GlaxoSmithKline. In the studies performed with this agent, it has been suggested that SB-269970 acts either as a selective antagonist or inverse agonist of the serotonin receptor 7 (5-HT7). |
| Molecular Formula |
C18H28N2O3S
|
|
|---|---|---|
| Molecular Weight |
352.49
|
|
| Exact Mass |
352.18
|
|
| Elemental Analysis |
C, 61.33; H, 8.01; N, 7.95; O, 13.62; S, 9.10
|
|
| CAS # |
201038-74-6
|
|
| Related CAS # |
SB-269970 hydrochloride; 261901-57-9
|
|
| PubChem CID |
6604889
|
|
| Appearance |
Solid powder
|
|
| Boiling Point |
512.9ºC at 760 mmHg
|
|
| Flash Point |
264ºC
|
|
| Vapour Pressure |
3.87E-11mmHg at 25°C
|
|
| LogP |
4.425
|
|
| Hydrogen Bond Donor Count |
1
|
|
| Hydrogen Bond Acceptor Count |
5
|
|
| Rotatable Bond Count |
5
|
|
| Heavy Atom Count |
24
|
|
| Complexity |
497
|
|
| Defined Atom Stereocenter Count |
1
|
|
| SMILES |
OC1=CC=CC(S(=O)(N2[C@@H](CCN3CCC(C)CC3)CCC2)=O)=C1
|
|
| InChi Key |
HWKROQUZSKPIKQ-MRXNPFEDSA-N
|
|
| InChi Code |
InChI=1S/C18H28N2O3S/c1-15-7-11-19(12-8-15)13-9-16-4-3-10-20(16)24(22,23)18-6-2-5-17(21)14-18/h2,5-6,14-16,21H,3-4,7-13H2,1H3/t16-/m1/s1
|
|
| Chemical Name |
3-[(2R)-2-[2-(4-methylpiperidin-1-yl)ethyl]pyrrolidin-1-yl]sulfonylphenol
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
30%propylene glycol+ 5%Tween 80+ 65%D5W: 30.0mg/ml (77.13mM) (Please use freshly prepared in vivo formulations for optimal results.)
|
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8370 mL | 14.1848 mL | 28.3696 mL | |
| 5 mM | 0.5674 mL | 2.8370 mL | 5.6739 mL | |
| 10 mM | 0.2837 mL | 1.4185 mL | 2.8370 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
 |
|---|
 |
 |
 |
|---|
 |