
| Size | Price | Stock | Qty |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g | |||
| Other Sizes |
Purity: ≥98%
Prucalopride (Motegrity; R-108512; R 108512; Resolor; Resotran; R108512; Prudac), a novel enterokinetic compound and the first representative drug of the benzofuran class of laxatives, is a selective and high affinity 5-HT (serotonin) receptor agonist with Kis of 2.5 nM and 8 nM for 5-HT4A and 5-HT4B receptor, respectively. It has a selectivity against other 5-HT receptor subtypes that is >290-fold higher. A pEC50 of 7.5 indicates that prucalopride causes contractions in a concentration-dependent manner. The rebound contraction of the proximal colon of guinea pigs following electrical field stimulation is markedly enhanced by practivopride. For the treatment of chronic idiopathic constipation, prucalopride received FDA approval in December 2018.
| Targets |
5-HT4A Receptor ( pKi = 8.6 ); 5-HT4B Receptor ( pKi = 8.1 )
|
|
|---|---|---|
| ln Vitro |
|
|
| ln Vivo |
|
|
| Enzyme Assay |
Prucalopride is the first member of the benzofuran class and a novel enterokinetic compound. Our goal was to determine its pharmacological profile through a range of organ bath and receptor binding experiments. With mean pK(i) estimates of 8.60 and 8.10 for the human 5-HT(4a) and 5-HT(4b) receptors, respectively, receptor binding data have shown prucaloprides high affinity to both investigated 5-HT(4) receptor isoforms. Only the human D(4) receptor (pK(i) 5.63), the mouse 5-HT(3) receptor (pK(i) 5.41) and the human sigma(1) (pK(i) 5.43) have demonstrated measurable affinity from the 50 other binding assays examined in this study, resulting in at least 290-fold selectivity for the 5-HT(4) receptor. Classical organ bath experiments were performed using different protocols on isolated gastrointestinal tract tissues from rats, guinea pigs, and dogs. In the guinea-pig colon, prucalopride was a 5-HT(4) receptor agonist because it caused contractions (pEC(50)=7.48+/-0.06; insensitive to a 5-HT(2A) or 5-HT(3) receptor antagonist, but inhibited by a 5-HT(4) receptor antagonist) and promoted noncholinergic contractions induced by electrical stimulation (blocked by a 5-HT(4) receptor antagonist). In addition, it resulted in the 5-HT(4) receptor antagonist-sensitive relaxation of a rat oesophagus preparation (pEC(50)=7.81+/-0.17). Up to 10 microM, pracilocapride did not significantly inhibit the contractions mediated by the 5-HT(2A), 5-HT(2B), or 5-HT(3) receptors, motilin or cholecystokinin (CCK(1)) receptors, or nicotinic or muscarinic acetylcholine receptors. In summary, prucalopride is a strong, particular, and selective 5-HT(4) receptor agonist. It is significant to note that prucalopride does not impede the actions of 5-HT(2A), 5-HT(2B), and 5-HT(3) receptors, motilin, or CCK(1) receptors, and is not anti-cholinergic, anticholinesterase, or nonspecific inhibitory activity. This is important because the drug is intended to treat disorders related to intestinal motility.
|
|
| Cell Assay |
Classical organ bath experiments were performed using different protocols on isolated gastrointestinal tract tissues from rats, guinea pigs, and dogs. In the guinea-pig colon, prucalopride was a 5-HT(4) receptor agonist because it caused contractions (pEC(50)=7.48+/-0.06; insensitive to a 5-HT(2A) or 5-HT(3) receptor antagonist, but inhibited by a 5-HT(4) receptor antagonist) and promoted noncholinergic contractions induced by electrical stimulation (blocked by a 5-HT(4) receptor antagonist). In addition, it resulted in the 5-HT(4) receptor antagonist-sensitive relaxation of a rat oesophagus preparation (pEC(50)=7.81+/-0.17).
|
|
| Animal Protocol |
Sprague dawley rats (diabetes mellitus (DM) model)
5 or 10 µg/kg Oral gavage; single daily for 2 weeks. |
|
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
Prucalopride is well absorbed and it reaches maximum plasma concentration of 3.79ng/ml with a tmax of 2.77 hours after initial administration. It presents an AUC of 96.5 mn.h/ml. The bioavailability of prucalopride is of over 90% and this bioavailability does not get influenced by the ingestion of food. After maximum plasma concentration, prucalopride concentration decline in a biphasic manner. Prucalopride is mainly excreted by the urine, representing 84% of the administered dose while only 13% of the dose is recovered in feces. The mean volume of distribution of prucalopride is registered to be 623 L. Prucalopride renal clearance is reported to be of 17 L/h which actually exceeds the glomerular filtration rate of the kidney. Metabolism / Metabolites Prucalopride is not extensively metabolized in the body and does not interact with the enzymes of the family of the cytochrome P450 enzymes nor the P glycoprotein. The metabolism of prucalopride only represents 6% of the administered dose and the remaining 94% is found as the unchanged drug. From studies, it was reported the recovery of 8 metabolites being the major metabolite R107504 which is formed after the O-demethylation and oxidation of the resulting alcohol to a carboxylic acid. Biological Half-Life The reported half-life of prucalopride is of around 18-20 hours. |
|
| Toxicity/Toxicokinetics |
Hepatotoxicity
Prucalopride therapy was associated with a low rate of serum enzyme elevations during therapy ( Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No published experience exists with prucalopride during breastfeeding. However, the manufacturer reports an unpublished study that indicates a relatively low amount of drug in breastmilk. Until more data become available, monitor the breastfed infant for diarrhea. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. ◈ What is prucalopride? Prucalopride is a medication used to treat adults with functional constipation, also known as chronic idiopathic constipation. Here, chronic means that symptoms, such as hard, infrequent bowel movements, have been happening for at least 6 months. Idiopathic means that the cause is not known. Prucalopride is sold under the brand name Motegrity®.Other forms of constipation might respond to over-the-counter (OTC) laxatives. For more information about OTC laxatives, please see the general laxatives MotherToBaby fact sheet at https://mothertobaby.org/fact-sheets/laxatives/.Sometimes when people find out they are pregnant, they think about changing how they take their medication or stopping their medication altogether. However, it is important to talk with your healthcare providers before making any changes to how you take this medication. Your healthcare providers can talk with you about the benefits of treating your condition and the risks of untreated illness during pregnancy. ◈ I take prucalopride. Can it make it harder for me to get pregnant? Studies have not been done to see if prucalopride could make it harder to get pregnant. ◈ Does taking prucalopride increase the chance for miscarriage? Miscarriage is common and can occur in any pregnancy for many different reasons. It is not known if prucalopride increases the chance of miscarriage, ◈ Does taking prucalopride increase the chance of birth defects? Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. It is not known if prucalopride increases the chance for birth defects above the background risk. Human studies have not been done to see if prucalopride increases the chance for birth defects. Animal studies done by the manufacturer did not show an increase in birth defects with exposure to prucalopride. ◈ Does taking prucalopride in pregnancy increase the chance of other pregnancy-related problems? Studies have not been done to see if prucalopride increases the chance for pregnancy-related problems such as preterm delivery (birth before week 37) or low birth weight(weighing less than 5 pounds, 8 ounces [2500 grams] at birth). ◈ Does taking prucalopride in pregnancy affect future behavior or learning for the child? Studies have not been done to see if prucalopride can cause behavior or learning issues for the child. ◈ Breastfeeding while taking prucalopride: There are no published studies looking at the use of prucalopride during breastfeeding. The manufacturer reports an unpublished study that indicates a relatively low amount of prucalopride is passed into breast milk. If you suspect the baby has any symptoms (such as diarrhea), contact the child’s healthcare provider. Be sure to talk to your healthcare provider about all of your breastfeeding questions. ◈ If a male takes prucalopride, could it affect fertility (ability to get partner pregnant) or increase the chance of birth defects? Studies have not been done to see if prucalopride could affect male fertility or increase the chance of birth defects. In general, exposures that fathers or sperm donors have are unlikely to increase the risks to a pregnancy. For more information, please see the MotherToBaby fact sheet Paternal Exposures at https://mothertobaby.org/fact-sheets/paternal-exposures-pregnancy/. Protein Binding The plasma protein binding of prucalopride is of 30%. |
|
| References | ||
| Additional Infomation |
Prucalopride is a member of benzamides.
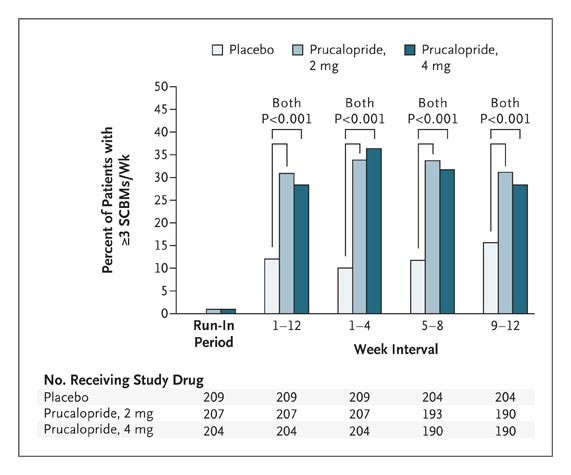
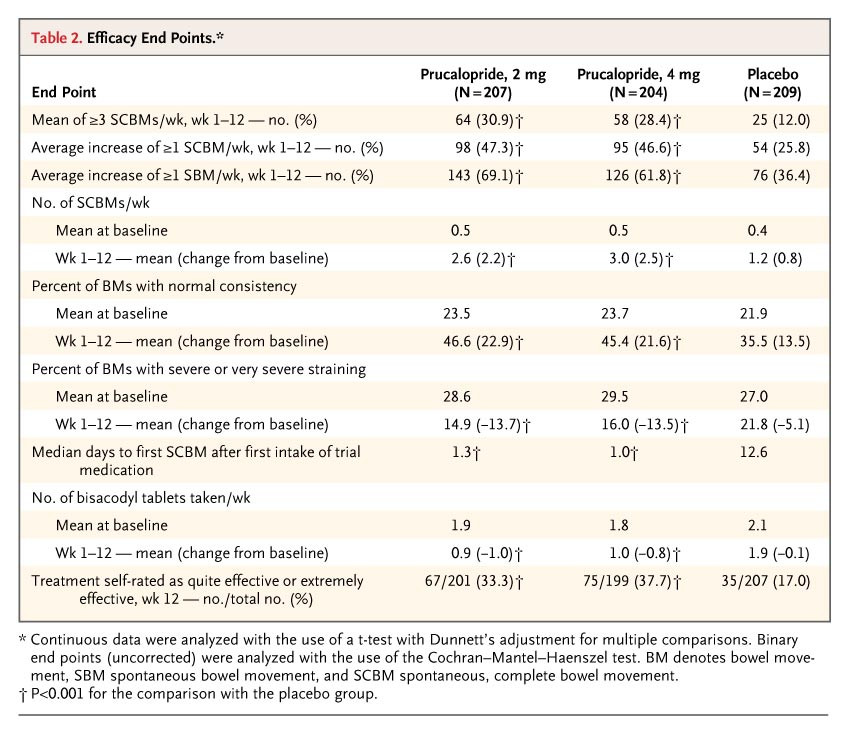
Prucalopride is a dihydrobenzofurancarboxamide derivative from the benzofurane family that selectively stimulates 5-HT4 receptors and thus, it presents enterokinetic properties. The high selectivity of prucalopride allowed further development as it prevented the cardiac adverse reactions observed due to non-target effects of precedent therapies. Prucalopride was developed by Shire Development LLC and approved for use in Europe in 2009, in Canada on December 7, 2011 and by the FDA on December 17, 2018. Prucalopride is a Serotonin-4 Receptor Agonist. The mechanism of action of prucalopride is as a Serotonin 4 Receptor Agonist. Prucalopride is a serotonin type 4 (5-HT4) receptor agonist that has potent prokinetic activity and is used as therapy for chronic idiopathic constipation. Prucalopride has been associated with a minimal rate of transient serum enzyme elevations during therapy and has not been implicated in cases of clinically apparent liver injury with jaundice. Prucalopride is an orally bioavailable dihydro-benzofuran-carboxamide and selective serotonin (5-HT4) receptor agonist, with gastrointestinal (GI) prokinetic activity. Upon oral administration, prucalopride specifically targets, binds to and stimulates the 5-HT4 receptor. This alters colonic motility patterns and stimulates colonic mass movements. This may normalize bowel movements and may relief chronic constipation. In addition, by increasing esophageal and gastric motility, prucalopride may also provide relief for aspiration-associated symptoms. See also: Prucalopride Succinate (active moiety of); Prucalopride hydrochloride (is active moiety of). Drug Indication Prucalopride is indicated for the treatment of chronic idiopathic constipation (CIC) in adults. CIC is one of the most common chronic functional gastrointestinal disorders worldwide. The diagnosis of this agent is very hard and it can be confirmed if the patient experience at least two of the following: -Straining during more than 25% of the bowel movements. -Lumpy or hard stools in 25% of the bowel movements. -Sensation of incomplete evacuation in more than 25% of all bowel movements. -Sensation of anorectal blockage or obstruction in more than 25% of the bowel movements. -Manual maneuvers required in more than 25% of the bowel movements. -Fewer than 3 bowel movements per week. FDA Label Resolor is indicated for symptomatic treatment of chronic constipation in adults in whom laxatives fail to provide adequate relief. Mechanism of Action Prucalopride acts as a selective stimulator of the 5-HT4 receptors while having no interaction with hERG channel or 5-HT1 receptors which reduces significantly the cardiovascular risk found in other similar drugs. 5-HT4 receptors can be found throughout the gastrointestinal tract primarily in smooth muscle cells, enterochromaffin cells, and myenteric plexus. Its activation produces the release of acetylcholine which is the major excitatory neurotransmitter in the GI tract. Hence, prucalopride stimulates motility by interacting specifically with 5-HT4 receptors in the GI tract which causes a release of acetylcholine and further contraction of the muscle layer of the colon and relaxation of the circular muscle layer leading to the propulsion of luminal content. Pharmacodynamics In animal studies, prucalopride induced a dose-dependent stimulation of contractile activity in the proximal colon and inhibition of the contractility in the distal colon. As well it has been shown that prucalopride stimulates and amplifies giant migratory contraction which is the high-amplitude type of contraction that initiates the urge to defecate. Thus, prucalopride not only accelerates the colonic transit but also accelerates gastric emptying and small bowel transit. In supratherapeutic concentrations, prucalopride can be observed to interact with hERG potassium channels and L-type calcium channels. In clinical trials, prucalopride showed to significantly increase the spontaneous bowel movements with a standardized mean difference of about 0.5 after the use of 1 mg when compared with the placebo group. In this studies as well, it was observed a numerical improvement in mean colonic transit time and a significant increase in spontaneous complete bowel movement without marked changes in the anorectal function. In phase III clinical trials, 86% of the tested individuals opted to continue with the open-label study and based on Patients Assessments, 67% of the patients increase more than one point improvement in their satisfaction. In the final set of clinical trials for approval, there was a significant increase in the number of patients that reached over 3 complete spontaneous bowel movements per week when compared with the placebo. |
| Molecular Formula |
C18H26CLN3O3
|
|---|---|
| Molecular Weight |
367.87
|
| Exact Mass |
367.166
|
| Elemental Analysis |
C, 58.77; H, 7.12; Cl, 9.64; N, 11.42; O, 13.05
|
| CAS # |
179474-81-8
|
| Related CAS # |
Prucalopride succinate; 179474-85-2; Prucalopride-13C,d3; 2140306-00-7; Prucalopride hydrochloride; 179474-80-7; 179474-85-2 (Succinate)
|
| PubChem CID |
3052762
|
| Appearance |
White to off-white solid powder
|
| Density |
1.3±0.1 g/cm3
|
| Boiling Point |
481.4±45.0 °C at 760 mmHg
|
| Melting Point |
90.7°
|
| Flash Point |
244.9±28.7 °C
|
| Vapour Pressure |
0.0±1.2 mmHg at 25°C
|
| Index of Refraction |
1.599
|
| LogP |
1.44
|
| Hydrogen Bond Donor Count |
2
|
| Hydrogen Bond Acceptor Count |
5
|
| Rotatable Bond Count |
6
|
| Heavy Atom Count |
25
|
| Complexity |
445
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
ClC1=C(C2C([H])([H])C([H])([H])OC=2C(=C1[H])C(N([H])C1([H])C([H])([H])C([H])([H])N(C([H])([H])C([H])([H])C([H])([H])OC([H])([H])[H])C([H])([H])C1([H])[H])=O)N([H])[H]
|
| InChi Key |
ZPMNHBXQOOVQJL-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C18H26ClN3O3/c1-24-9-2-6-22-7-3-12(4-8-22)21-18(23)14-11-15(19)16(20)13-5-10-25-17(13)14/h11-12H,2-10,20H2,1H3,(H,21,23)
|
| Chemical Name |
4-amino-5-chloro-N-[1-(3-methoxypropyl)piperidin-4-yl]-2,3-dihydro-1-benzofuran-7-carboxamide
|
| Synonyms |
Prucalopride free base; R 093877; R-093877; R093877; R-108512; R 108512; R108512; R-93877; R93877; Prucalopride; Motegrity; Resolor; Resotran; R 93877
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: 50 mg/mL (135.92 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with sonication.
Solubility in Formulation 2: ClC1=C(N)C(CCO2)=C2C(C(NC3CCN(CCCOC)CC3)=O)=C1 (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7184 mL | 13.5918 mL | 27.1835 mL | |
| 5 mM | 0.5437 mL | 2.7184 mL | 5.4367 mL | |
| 10 mM | 0.2718 mL | 1.3592 mL | 2.7184 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
Prucalopride and Cognition in Recovered Depression
CTID: NCT05220228
Phase: N/A Status: Completed
Date: 2024-02-09
 |
|---|
 |
 |