| Size | Price | Stock | Qty |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
Purity: =98.60%
| Targets |
Acetylcholinesterase
|
|---|---|
| ln Vitro |
Protopine (10–40 μM, 24–96 h) limits the ability of translocated cells (HepG2, Huh7) to migrate, proliferate, and undergo EMT [2]. Protopine (10–40 μM, 24 h) suppresses PI3K/Akt signaling dye and causes cell opening in HepG2 and Huh7 cells via expressing caspase-3 and caspase-9 [2]. Protopine (0–10 μg/mL) inhibits serotonin transporter (SERT) function in S6 cells and N1 cells, while protopine (10–40 μM, 6 hours) increases the formation of reactive oxygen species (ROS) in HepG2 and Huh7 cells [3].
|
| ln Vivo |
Protopine (0.1 and 1 mg/kg, intraperitoneal injection) can attenuate scopolamine (1 mg/kg)-induced memory impairment in mice [1]. Protropine (5-20 mg/kg, i.v.) inhibits tumor growth and inhibits PI3K/Akt, and induces caspase-3 channels in xenografted BALB/c mice (subcutaneously injected with HepG2 or Huh-7 cells) [ 2]. Pratopine (5-20 mg/kg, intraperitoneally) shows antidepressant-like effects in mouse HTR and TST tests [3]. Protopine (1-4 mg/kg, intraperitoneally, once daily for 3 days) has a protective effect against focal brain injury [4].
|
| Enzyme Assay |
Protopine is an isoquinoline alkaloid that possesses various biological activities including the anti-tumour activity. However, the effects of protopine on liver carcinoma cells are still elusive. The aim of this study is to examine the effects of protopine on liver carcinoma cells both in vitro and in vivo.
Methods: MTT assay was performed to measure the cell viability. Wound healing and transwell assays were conducted to assess the motility of cells. Cellular apoptosis and ROS levels were measured by the flow cytometry. Western blotting assay was used to measure the change of proteins. The cytotoxicity of protopine was also evaluated in xenograft mice.
Results: Protopine inhibited viabilities and triggered apoptosis via the intrinsic pathway in a caspase-dependent manner in liver carcinoma cells. Furthermore, protopine also induced accumulation of intracellular ROS which further led to the inhibition of PI3K/Akt signalling pathway. Finally, in vivo study showed that protopine also repressed tumour growth in xenograft mice without noticeable toxicity.
Conclusions: Protopine might be used as a potential therapeutic agent for the treatment of liver carcinoma[2].
|
| Cell Assay |
Western Blot Analysis[2]
Cell Types: HepG2, Huh7 Tested Concentrations: 10, 20, 40 μM Incubation Duration: 24 hrs (hours) Experimental Results: Induced caspase-3 and caspase-9 cleavage. Bcl-2 and Bcl-xl levels are diminished. Induces the release of the mitochondrial protein cytochrome c into the cytoplasm. |
| Animal Protocol |
Animal/Disease Models: 5-Hydroxy-DL-tryptophan (5-HTP)-induced mouse model [3]
Doses: 5, 10, 20 mg/kg Route of Administration: intraperitoneal (ip) injection Experimental Results: Increased 5-HTP-induced head Number of hemispheric twitch responses (HTR). Reduce the immobility time tested in the Tail Suspension Test (TST). The protopine isolated from a Chinese herb Dactylicapnos scandens Hutch was identified as an inhibitor of both serotonin transporter and noradrenaline transporter in vitro assays. 5-hydroxy-DL-tryptophan(5-HTP)-induced head twitch response (HTR) and tail suspension test were adopted to study whether protopine has anti-depression effect in mice using reference antidepressant fluoxetine and desipramine as positive controls. In HTR test, protopine at doses of 5, 10, 20 mg/kg dose dependently increase the number of 5-HTP-induced HTR. Protopine at doses of 3.75 mg/kg, 7.5 mg/kg and 30 mg/kg also produces a dose-dependent reduction in immobility in the tail suspension test. The present results open up new possibilities for the use of protopine in the treatment of mood disorders, such as mild and moderate states of depression.[3] Protopine, an isoquinoline alkaloidis, is known to produce many effects such as vasodilation, down-regulation of glutamate levels in brain and decrease of intracellular calcium. However, so far there is no report on the effect of protopine in cerebral ischaemia. In this study, the effect of protopine on the focal cerebral ischaemia was investigated in rats. Male Sprague-Dawley rats were divided into five groups: sham-operated group, vehicle-treated group and three doses of protopine-treated groups (0.98, 1.96 and 3.92 mg/kg). Protopine was intraperitoneally administered to rats once daily for 3 days prior to the ischaemia and 0.9% normal saline to rats in the vehicle-treated group in the same pattern. Rats in the sham-operated group were given 0.9% normal saline without the ischaemia. The focal cerebral ischaemia was induced by the middle cerebral artery occlusion for 24 hr via the intraluminal filament technique. The results showed that pre-treatment with protopine reduced the cerebral infarction ratio and serum lactate dehydrogenase activity, and improved the ischaemia-induced neurological deficit score and histological changes of brain in a dose-dependent manner. The further studies demonstrated that protopine increased superoxide dismutase activity in serum, and decreased total calcium and terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL)-positive cells in the ischaemic brain tissue in the middle cerebral artery occlusion rats. The results indicate that protopine is able to produce an effective protection on the injury caused by the focal cerebral ischaemia in rats possibly through the multiple effects of calcium antagonism, antioxidation and depression of cell apoptosis.[4] |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
Xiang-Fu-Si-Wu Decoction (XFSWD) has been widely used to treat primary dysmenorrhea in clinical practice for hundreds of years and shown great efficacy. One fraction of XFSWD, which was an elution product by macroporous adsorption resin from aqueous extract solution with 60% ethanol (XFSWE), showed great analgesic effect. The present study was conducted to investigate the possible pharmacokinetic and tissue distribution profiles of four major bioactive constituents (berberine, protopine, tetrahydrocoptisine and tetrahydropalmatine) after oral administration of XFSWE in dysmenorrheal symptom rats, and to compare the difference between normal and dysmenorrheal symptom rats. Estradiol benzoate and oxytocin were used to produce dysmenorrheal symptom rat model. The experimental period was seven days. At the final day of experimental period, both normal and dysmenorrheal symptom rats were orally administrated with XFSWE, and then the blood and tissues samples were collected at different time points. Berberine, protopine, tetrahydrocoptisine and tetrahydropalmatine in blood and tissue samples were determined by LC-MS/MS. Pharmacokinetic parameters were calculated from the plasma concentration-time data using non-compartmental methods. The differences of pharmacokinetic parameters among groups were tested by one-way analysis of variance (ANOVA). There were statistically significant differences (P<0.05) in Cmax, Tmax, AUC(0-t), AUC(0-infinity), MRT(0-t), MRT(0-infinity) and CL/F between normal and dysmenorrheal symptom rats that orally administered with same dosage of XFSWE. In tissue distribution study, the results showed that the overall trend was C(Spleen)>C(Liver)>C(Kidney)>C(Uterus)>C(Heart)>C(Lung)>C(Ovary)>C(Brain)>C(Thymus), C(M-60 min)>C(M-120 min)>C(M-30 min)>C(C-60 min)>C(C-120 min)>C(C-30 min). The contents of protopine in liver, spleen and uterus were more than that in other tissues of dysmenorrheal symptom rats. Compared to normal rats, partial contents of the compounds in dysmenorrheal symptom rats' tissues at different time points had significant difference (P<0.05). This study was the first report about pharmacokinetic and tissue distribution investigation in dysmenorrheal symptom animals. The results indicated that berberine, protopine, tetrahydrocoptisine and tetrahydropalmatine have higher uptake and slower elimination in the rats with dysmenorrheal syndrome, which suggests that the rate and extent of drug metabolism were altered in dysmenorrheal syndrome rats. And the results also demonstrated that berberine, protopine and tetrahydropalmatine in normal and dysmenorrheal symptom rats had obvious differences in some organs and time points, suggesting that the blood flow and perfusion rate of the organ were altered in dysmenorrheal symptom animals. Metabolism / Metabolites Eschscholtzia californica preparations are in use as phytopharmaceuticals and as herbal drugs. Studies are described on the metabolism and the toxicological analysis of the Eschscholtzia californica alkaloids californine and protopine in rat urine using gas chromatography-mass spectrometry. ... Protopine ... undergoes extensive demethylenation of the 2,3-methylenedioxy group followed by catechol-O-methylation. All phenolic hydroxy metabolites were found to be partly conjugated. The authors' systematic toxicological analysis procedure using full-scan gas chromatography-mass spectrometry after acid hydrolysis, liquid-liquid extraction and microwave-assisted acetylation allowed the detection of the main metabolites of californine and protopine in rat urine after a dose which should correspond to that of drug users. Therefore, use of Eschscholtzia californica preparations should also be detectable in human urine by the authors' systematic toxicological analysis procedure. |
| Toxicity/Toxicokinetics |
Toxicity Summary
IDENTIFICATION AND USE: Protopine is a solid. It is used as medication. HUMAN EXPOSURE AND TOXICITY: Using gene reporter assays performed in transiently transfected HepG2 cells, it was demonstrated that the induction of CYP1A1 expression by protopine was associated with mild or negligible activation of the aryl hydrocarbon receptor. CYP1A mRNA levels induced by protopine in both HepG2 cells and human hepatocytes did not result in elevated CYP1A protein or activity levels. ANIMAL STUDIES: Protopine showed an ability to enhance gamma-aminobutyric acid binding to rat brain synaptic membrane receptors in in vitro radiolabeling studies. Protopine has antiarrhythmic effects and may directly inhibit rapid electrical activity of cardiac cells. Protopine has been found to inhibit histamine H1 receptors and platelet aggregation, and acts as an analgesic. It is one of the compounds with activity like OPIATE ALKALOIDS, acting at OPIOID RECEPTORS. Properties include induction of ANALGESIA or NARCOSIS. Protopine can selectively bind to but do not activate histamine H1 receptors, thereby blocking the actions of endogenous histamine. Classical antihistaminics antagonize or prevent the action of histamine mainly in immediate hypersensitivity. They act in the bronchi, capillaries, and some other smooth muscles, and are used to prevent or allay motion sickness, seasonal rhinitis, and allergic dermatitis and to induce somnolence. Protopine can also function as platelet aggregation inhibitors which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. Protopine inhibits the contractility of isolated cardiac papillary muscles and the proliferation of vascular smooth muscle cells induced by endothelin. It also shortens action potential duration and prolongs the effective refractory period in guinea pig cardiac papillary muscles. The protective effect on rat heart from ischemia_reperfusion damage and the relaxation of rat thoracic aorta induced by protopine have been related to the inhibition of Ca2+ influx through both voltage- and receptor-operated Ca2+ channels. Protopine has been the focus of a large number of biological studies in which they both exhibited, for instance, anti-parasitic activity and only weak cytotoxicity in comparison with other types of isoquinoline alkaloids. Protopine was found to be cytoprotective against oxidative stress induced cell death in vitro. The alkaloid was shown to have anti-arrhythmic, anti-thrombotic, anti-inflammatory, and hepatoprotective effects in animal models. The biological activity of protopine may be associated with its ability to inhibit calcium, sodium, and potassium channels. (PMID:15588728; PMID:21419197; L2104) Interactions The antiarrhythmic effects of protopine on experimental arrhythmia were studied in various animals. Protopine elevated the dose of aconitine needed to induce VP, VT, and VF in rats and increased the dose of strophanthin (strophanthine K) that induced VP in guinea pigs. It also shortened the duration of central arrhythmia induced by aconitine and the duration of arrhythmia induced by benzene-epinephrine (adrenaline) in rats. It prevented rats and mice from developing arrhythmia induced by intravenous calcium chloride and inhalation of chloroform, respectively. In rabbits, the drug raised VFT. It was concluded that protopine has antiarrhythmic effects and may directly inhibit rapid electrical activity of cardiac cells. Non-Human Toxicity Values LD50 Guinea pig ip 116 mg/kg LD50 Guinea pig oral 237 mg/kg LD50 Mouse ip 482 mg/kg |
| References |
|
| Additional Infomation |
Protopine is a dibenzazecine alkaloid isolated from Fumaria vaillantii. It has a role as a plant metabolite.
Protopine has been reported in Corydalis solida, Corydalis ternata, and other organisms with data available. Protopine is a benzylisoquinoline alkaloid occurring in opium poppies and other plants of the family papaveraceae. It has been found to inhibit histamine H1 receptors and platelet aggregation, and acts as an opioid analgesic. See also: Sanguinaria canadensis root (part of); Chelidonium majus flowering top (part of). Therapeutic Uses Analgesics, Opioid; Histamine H1 Antagonists; Platelet Aggregation Inhibitors /EXPL THER/ ... In the present study, we studied the anticancer proliferation and adhesion effects of five alkaloids which were isolated from Corydalis yanhusuo. MTT dose response curves, cell migration assay, cell invasion assay, as well as three types of cell adhesive assay were performed on MDA-MB-231 human breast cancer cells. The mechanism of the compounds on inhibiting heterotypic cell adhesion were further explored by determining the expression of epidermal growth factor receptor (EGFR), Intercellular adhesion molecule 1 (ICAM-1), alphav-integrin, beta1-integrin and beta5-integrin by western blotting assay. In five tested alkaloids, only protopine exhibited anti-adhesive and anti-invasion effects in MDA-MB-231 cells, which contributed to the anti-metastasis effect of Corydalis yanhusuo. The results showed that after treatment with protopine for 90 min, the expression of EGFR, ICAM-1, alphav-integrin, beta1-integrin and beta5-integrin were remarkably reduced. The present results suggest that protopine seems to inhibit the heterotypic cell adhesion between MDA-MB-231 cells, and human umbilical vein endothelial cells by changing the expression of adhesive factors. /EXPL THER/ ... In this study, we investigated whether protopine derived from Hypecoum erectum L could suppress lipopolysaccharide (LPS)-induced inflammatory responses in murine macrophages (Raw 264.7 cells). Protopine was found to reduce nitric oxide (NO), cyclooxygenase-2 (COX-2), and prostaglandin E(2) (PGE(2)) production by LPS-stimulated Raw 264.7 cells, without a cytotoxic effect. Pre-treatment of Raw 264.7 cells with protopine reduced the production of pro-inflammatory cytokines. These inhibitory effects were caused by blocking phosphorylation of mitogen-activated protein kinases (MAP kinases) and also blocking activation of a nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kappaB). /EXPL THER/ In this study, we investigated the anticancer effect of protopine on human hormone-refractory prostate cancer (HRPC) cells. Protopine exhibited an anti-proliferative effect by induction of tubulin polymerization and mitotic arrest, which ultimately led to apoptotic cell death. The data suggest that protopine increased the activity of cyclin-dependent kinase 1 (Cdk1)/cyclin B1 complex and that contributed to cell apoptosis by modulating mitochondria-mediated signaling pathways, such as Bcl-2 phosphorylation and Mcl-1 down-regulation. In conclusion, the data suggest that protopine is a novel microtubule stabilizer with anticancer activity in HRPC cells through apoptotic pathway by modulating Cdk1 activity and Bcl-2 family of proteins. For more Therapeutic Uses (Complete) data for PROTOPINE (12 total), please visit the HSDB record page. |
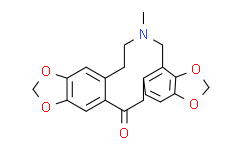
| Molecular Formula |
C20H19NO5
|
|---|---|
| Molecular Weight |
353.374
|
| Exact Mass |
353.126
|
| CAS # |
130-86-9
|
| Related CAS # |
Protopine hydrochloride;6164-47-2
|
| PubChem CID |
4970
|
| Appearance |
White to off-white solid powder
|
| Density |
1.3±0.1 g/cm3
|
| Boiling Point |
547.5±49.0 °C at 760 mmHg
|
| Melting Point |
211ºC
|
| Flash Point |
284.9±29.8 °C
|
| Vapour Pressure |
0.0±1.5 mmHg at 25°C
|
| Index of Refraction |
1.613
|
| LogP |
3.76
|
| Hydrogen Bond Donor Count |
0
|
| Hydrogen Bond Acceptor Count |
6
|
| Rotatable Bond Count |
0
|
| Heavy Atom Count |
26
|
| Complexity |
542
|
| Defined Atom Stereocenter Count |
0
|
| InChi Key |
GPTFURBXHJWNHR-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C20H19NO5/c1-21-5-4-13-7-18-19(25-10-24-18)8-14(13)16(22)6-12-2-3-17-20(15(12)9-21)26-11-23-17/h2-3,7-8H,4-6,9-11H2,1H3
|
| Chemical Name |
15-methyl-7,9,19,21-tetraoxa-15-azapentacyclo[15.7.0.04,12.06,10.018,22]tetracosa-1(17),4,6(10),11,18(22),23-hexaen-3-one
|
| Synonyms |
Fumarine; Biflorine; Corydinine; Fumarine; Biflorine; Macleyine; Protopin; Hypercorine; Protopine
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
DMSO : ~12.5 mg/mL (~35.37 mM)
|
|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 1.47 mg/mL (4.16 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 14.7 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 1.25 mg/mL (3.54 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 12.5 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 1.25 mg/mL (3.54 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8299 mL | 14.1495 mL | 28.2990 mL | |
| 5 mM | 0.5660 mL | 2.8299 mL | 5.6598 mL | |
| 10 mM | 0.2830 mL | 1.4149 mL | 2.8299 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
|
|
|