
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
Purity: ≥98%
Pictilisib (also called GDC-0941, Pictrelisib, RG-7321 and GNE-0941) is a potent and orally bioavailable inhibitor of PI3Kα/δ (class I phosphatidylinositol 3 kinase) with IC50 of 3 nM in cell-free assays. It has the potential to be anti-cancer and demonstrated a modest level of selectivity against p110 (11-fold) and p110 (25-fold). Tumorigenesis is frequently linked to the activation of the PI3K/Akt signaling pathway. This pathway is frequently dysregulated in a variety of cancers, which may play a role in the resistance to many anticancer drugs. The creation of novel small molecules that specifically block the PI3K/Akt pathway might stop the growth of tumors. Phosphatidylinositol-3, 4, 5-triphosphate (PIP3) is a second messenger that carries PI3K downstream signals, and GDC-0941 is made to bind to the ATP-binding pocket of PI3K and prevent its synthesis. It engages in ATP-competitive binding to PI3K.
| Targets |
p110α (IC50 = 3 nM); p110β (IC50 = 33 nM); p110δ (IC50 = 3 nM); p110γ (IC50 = 75 nM); p110α-H1047R (IC50 = 3 nM); p110α-E545K (IC50 = 3 nM); DNA-PK (IC50 = 1.23 μM); mTOR (Ki = 0.58 μM); Autophagy
|
|---|---|
| ln Vitro |
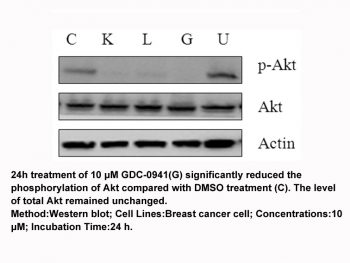
Pictilisib (GDC-0941) and RP-56976 reduce tumor cell viability by 80% or greater in the breast cancer cell lines than single-agent treatment. In the tumor models Hs578T1.2 (PI3K wild-type), MCF7-neo/HER2 (PI3K-mutant), and MX-1 (PTEN-null), GDC-0941 inhibits Akt phosphorylation as well as Akt signaling's downstream targets, such as pPRAS40 and pS6. The duration of RP-56976-induced mitotic arrest before apoptosis is shortened by pictilisib (GDC-0941)[1]. Pictilisib (GDC-0941) shows a high efficacy of antitumor activity in two ZD1839-resistant non-small cell lung cancer (NSCLC) cell lines, A549 and H460. Pictilisib (GDC-0941) is highly effective when combined with U0126 for inhibiting cell growth, G0-G1 arrest, and cell apoptosis. Pictilisib (GDC-0941) is relatively more toxic to A549 cells with wild-type PIK3CA than to H460 cells with activating mutations of PIK3CA[3]. As evidenced by a decline in pAK, pictilisib (GDC-0941) decreases PI3K pathway activity in both cell lines. Following hypoxic/anoxic exposure, pictilisib (GDC-0941) significantly lowers the amount of secreted VEGF that is found in the medium in all cells[4].
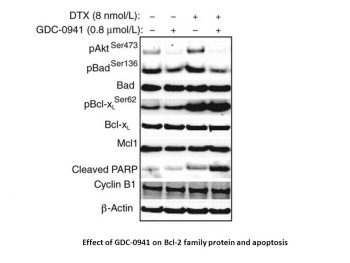
Combination of GDC-0941 and docetaxel decreased the cellular viability of breast tumor cell lines in vitro but to variable degrees of drug synergy. Compared with nontransformed MCF10A cells, the addition of both drugs resulted in stronger synergistic effects in a subset of tumor cell lines that were not predicted by breast cancer subtype. [1] Lung cancer is a malignant disease with poor outcome, which has led to a search for new therapeutics. The PI3K/Akt/mTOR and Ras/raf/Erk pathways are key regulators of tumor growth and survival. In the present study, their roles were evaluated by MTT assay, flow cytometry and Western blotting in lung cancer cells. We found that a high efficacy of antitumor activity was shown with GDC-0941 treatment in two gefitinib-resistant non-small cell lung cancer (NSCLC) cell lines, A549 and H460. In addition, H460 cells with activating mutations of PIK3CA were relatively more sensitive to GDC-0941 than A549 cells with wild-type PIK3CA. Furthermore, GDC-0941 was highly efficacious in combination with U0126 in inducing cell growth inhibition, G0-G1 arrest and cell apoptosis. These antitumor activities of combined treatment may be attributed to the alterations of G0-G1 phase regulators, apoptosis-related proteins and eukaryotic translation initiation factor 4B (eIF4B), induced by concomitant blockade of the PI3K/Akt/mTOR and Ras/raf/Erk pathways. In conclusion, this study suggests that multi‑targeted intervention is the most effective treatment for tumors. Additionally, the blockade of PI3K, mTOR and Erk with GDC-0941 and MEK inhibitors shows promise for treating gefitinib-resistant NSCLC [3]. |
| ln Vivo |
Pictilisib (GDC-0941) (150 mg/kg, p.o.) leads to tumor stasis in MCF7-neo/HER2-bearing animals model. Pictilisib (GDC-0941) and RP-56976 result in tumor regressions during the treatment period leading to enhanced antitumor responses[1]. When Pictilisib (GDC-0941) treatment is stopped, the test cohort mice's tumors grow once more[2]. Tumors in the Pictilisib (GDC-0941)-treated mice exhibit a notable non-linear shrinkage. Pictilisib (GDC-0941) (25 or 50 mg/kg) reduces tumor growth and PI3K and HIF-1 pathway activity in eGFP-FTC133 tumor-bearing mice[4].
Combination of GDC-0941 and docetaxel leads to enhanced antitumor efficacy and apoptosis in vivo [1] To confirm our in vitro observations that the combination of GDC-0941 with docetaxel led to increased tumor growth inhibition, we evaluated tumor xenograft models that were either PI3Kα-mutant (MCF7-neo/HER2), PTEN-null (MX-1) or PI3Kα wild-type (MAXF1162). Treatment of animals bearing MCF7-neo/HER2 breast cancer xenografts with 7.5 mg/kg docetaxel or 150 mg/kg GDC-0941 led to tumor growth delay and tumor stasis, respectively (Fig. 4A). In contrast, the combination of GDC-0941 and docetaxel resulted in tumor regressions during the treatment period leading to enhanced antitumor responses (Fig. 4A). Single-agent and combination treatments were at maximum tolerated doses based on minimal changes in animal body weights (Fig. 4B). Similar to the MCF7-neo/HER2 xenograft model, we observed greater than additive effects when GDC-0941 was administered in combination with docetaxel in the MX-1 xenograft model resulting in increased tumor regressions during the treatment period (Fig. 4C). Because the Hs578T1.2 tumor cell line is nontumorigenic in vivo, we evaluated the MAXF1162 patient–derived breast tumor xenograft model, which is HER2+/ER+/PR+, PI3Kα wild-type, and PTEN-positive (Oncotest Inc; personal communication). Treatment of MAXF1162 primary breast tumor xenografts with 15 mg/kg docetaxel in vivo as a single agent resulted in tumor growth delay (Fig. 4D). However, the combination of 100 mg/kg GDC-0941 and docetaxel resulted in tumor stasis during the treatment period that was sustained after dosing ended (Fig. 4D). GDC-0941 and docetaxel were administered at maximum tolerated doses, and no additional change in animal body weights were noted when both drugs were combined (data not shown). Dosing schedule of GDC-0941 in combination with docetaxel in vivo [1] Given that PI3K activity has been described to be necessary for progression through G1, S-, and G2 phases of the cell cycle, we determined whether the combination effects of GDC-0941 and docetaxel were dependent on the order of drug treatment. Increased apoptosis was detected when docetaxel was dosed 1 to 4 hours before GDC-0941 compared with docetaxel or GDC-0941 alone (Fig. 5A). Similarly, when docetaxel was dosed 4 hours before GDC-0941, an increase in the sub-G1 cell population occurred indicative of increased cell death (Supplementary Fig. S4). However, increased apoptosis compared with docetaxel alone was not observed when GDC-0941 was dosed 4 hours before docetaxel (Fig. 5A). Antitumor Activity [10] One patient with BRAF V600E-mutated metastatic melanoma, but no detected PI3K pathway deregulation, achieved a confirmed RECIST-partial response; she received pictilisib at 330mg once-daily (21/28 schedule) for 9.5 months. She had previously been treated sequentially with paclitaxel and dacarbazine, but had not received a BRAF or MEK inhibitor. A heavily-pretreated, platinum-refractory advanced epithelial ovarian cancer patient with PIK3CA amplification (with high polysomy and >60% of tumor cells harboring 4 copies of PIK3CA) and loss of PTEN achieved radiological stable disease for 4 months with GCIG-CA125 partial response13 (Supplementary Figure 3) associated with 36% reduction in SUV on 18max F-FDG-PET and 56% reduction in tumor phospho-S6 expression. A patient with cKIT exon 9 mutant gastrointestinal stromal tumor but no evidence of PI3K pathway deregulation achieved stable disease for 7.5 months on pictilisib 450mg once-daily, and this was associated with pharmacodynamic changes of 47% reduction in SUV on 18max F-FDG-PET and 75% reduction in tumor phospho-S6 expression. Of 60 patients, 12 (20%) remained on study for >3 months and 2 (3%) for >6 months. Supplementary Table 2 shows the pharmacokinetic-pharmacodynamic-clinical relationship of patients who demonstrated a partial response by RECIST, GCIG-CA125 or 18F-FDG-PET EORTC criteria. |
| Enzyme Assay |
Recombinant human PI3Kα, PI3Kβ, and PI3Kδ are coexpressed in a Sf9 baculovirus system with the p85α regulatory subunit and purified as GST-fusion proteins using affinity chromatography on glutathione-sepharose. Monomeric GST-fusions are used for both the expression and purification of recombinant human PI3Kγ . GDC-0941 is dissolved in DMSO and added to 20 mM Tris-HCl (pH 7.5) containing 200 μg yttrium silicate (Ysi) polylysine SPA beads, 4 mM MgCl2, 1 mM dithiothreitol (DTT), 1 μM ATP, 0.125 μCi [γ-33P]-ATP, and 4% (v/v) DMSO in a total volume of 50 μL. The kinase reaction is started in the assay mixture by adding the recombinant GST-fusion of PI3Kα (5 ng), PI3Kβ (5 ng), PI3Kδ (5 ng), or PI3Kγ (5 ng). The kinase reaction is stopped with 150 μL PBS following an hour of incubation at room temperature. It is then read using a Wallac Microbeta counter after being centrifuged for 2 minutes at 2000 rpm. A sigmoidal, dose-response curve fit in MDL Assay Explorer is used to determine the reported IC50 values.
|
| Cell Assay |
GDC-0941 is applied to cells for 48 and 72 hours at different concentrations. The CellTiter-Glo Luminescent Cell Viability Assay is used to identify cell viability and proliferation. By using a western blot, the pAkt (Ser473), cleaved caspase-3, and cleaved PARP are all examined. Apoptosis and caspase 3/7 activity are both detected using the Cell Death Detection ELISAplus assay and the Caspase-Glo 3/7 assay, respectively.
Cell viability assay [1] All drug treatments were tested in quadruplicate during a 4-day incubation period, and the relative number of viable cells was estimated using CellTiter-Glo. Total luminescence was measured on a Wallac Multilabel Reader. Cells were treated simultaneously with docetaxel (dose range = 0.0003–0.020 μmol/L) or GDC-0941 (dose range = 0.083–5 μmol/L) in an 8 × 10 matrix of concentrations chosen to encompass clinically relevant doses. The concentration of drug resulting in 50% maximal effective concentration (EC50) was determined using Prism software. Combination synergy of GDC-0941 and docetaxel was determined by Bliss independence analyses. A Bliss expectation for a combined response (C) was calculated by the equation: C = (A + B) − (A × B) where A and B are the fractional growth inhibitions of drug A and B at a given dose. The difference between the Bliss expectation and the observed growth inhibition of the combination of drugs A and B at the same dose is the “Delta.Bliss.” Delta.Bliss scores were summed across the dose matrix to generate a Bliss sum. Bliss sum = 0 indicates that the combination treatment is additive (as expected for independent pathway effects); Bliss sum > 0 indicates activity greater than additive (synergy); and Bliss sum < 0 indicates the combination is less than additive (antagonism). Statistical analysis comparing the Bliss sums for each cell line was conducted by the Student t test. Western blotting [1] Cells were treated at EC50 concentrations of GDC-0941, docetaxel, or both for 4 or 24 hours and lysed in 1× Cell Extraction Buffer supplemented with protease inhibitors and Phosphatase Inhibitor Cocktails 1 and 2. Protein concentrations were determined using the Pierce BCA Protein Assay Kit. For immunoblots, equal amounts of protein were separated by electrophoresis through NuPAGE Bis-Tris 10% gradient gels, transferred onto polyvinylidene difluoride membranes using the Criterion system, and probed with monospecific primary antibodies. Specific antigen–antibody interactions were detected with IRDye 680 or IRDye 800 infrared secondary antibodies using a LI-COR imaging system. FACS analysis [1] For cell-cycle analysis, cells were treated with EC50 concentrations of GDC-0941 and/or docetaxel for 24 hours, fixed in 100% ice-cold ethanol, and incubated in propidium iodide (PI) solution for 30 minutes and analyzed with a FACScan flow cytometer. For cell death analyses, cells were incubated with GDC-0941, docetaxel, or both drugs for 48 hours, stained with Annexin V-FITC and PI solution according to the manufacturer's instructions and analyzed with a FACScan flow cytomete. Time lapse microscopy imaging [1] Cells were seeded onto glass-bottom 24-well plates and, 24 hours later, incubated with drug-containing media. Cells were treated with EC50 concentrations of GDC-0941 and/or docetaxel, multiple fields per condition were selected, and fluorescent and phase-contrast images were recorded with a 10× objective every 15 minutes for 72 hours on an AxioObserver inverted microscope, equipped with an environmental chamber, MS2000 XY stage, and a CoolSNAP CCD camera. Mitotic events and cell death were scored as previously described. Cells were also cotreated with caspase inhibitor Z-VAD-FMK at a final concentration of 2 μmol/L. Statistical analysis was conducted by the Student t test. |
| Animal Protocol |
Female nu/nu mice are inoculated subcutaneously with MCF7-neo/HER2 or MX-1 breast cancer cells. Animals are distributed into groups of 10 animals each when tumors reach a mean volume of 200 to 250 mm3. Group sizes are determined by size matching. Once a week, intravenous RP-56976, a formulation of 3% EtOH and 97% saline, is given. Pictilisib (GDC-0941), a daily oral dose of MCT (0.5% methylcellulose, 0.2% Tween-80), is administered. By directly implanting tumors from patients under the skin of NMRI nu/nu mice, the MAXF1162 HER2+/ER+/PR+ patient-derived breast cancer tumor xenograft model was created. Volume of the tumor is calculated. Throughout a study, tumor size measurements are taken twice a week.
In vivo xenograft models [1] Female nu/nu mice were inoculated subcutaneously with MCF7-neo/HER2 or MX-1 breast cancer cells. When tumors reached a mean volume of 200 to 250 mm3, animals were size-matched and distributed into groups consisting of 10 animals per group. Docetaxel formulated in 3% EtOH, 97% saline was administered intravenously once weekly. GDC-0941, formulated in MCT (0.5% methylcellulose, 0.2% Tween-80) was dosed orally and daily. MAXF1162 is an HER2+/ER+/PR+ patient-derived breast cancer tumor xenograft model established at Oncotest, Inc., by directly implanting tumors subcutaneously from patient to NMRI nu/nu mice. Tumor volume was calculated as follows: tumor size (mm3) = (longer measurement × shorter measurement2) × 0.5. Tumor sizes were recorded twice weekly over the course of a study. Following data analysis, P values were determined using the Dunnett t test. For pharmacodynamic studies, tumor samples (n = 4) were immediately frozen or fixed in 10% neutral-buffered formalin. Tumors were dissociated in cell extraction buffer, and lysates were analyzed by Western blotting as described above. Immunohistochemistry was conducted using 5-μm paraffin sections of formalin-fixed tissue on a Ventana Benchmark XT instrument (VMSI) by deparaffinization, treatment with antigen retrieval buffer (VMSI), and incubation with anti-cleaved caspase-3 primary antibody (Cell Signaling Technology) at 37°C. Bound antibody was detected using DABMap technology (VMSI), and sections were counterstained with hematoxylin. Ten Pten+/−Lkb1+/hypo mice bearing tumours, ranging in age from 7 to 9.5 months and weights from 25 to 30 g, were divided into two groups: the test group (n=6) were given PI3K inhibitor GDC-0941 (GDC-0941 bismesylate at 75 mg/kg), whilst the control group (n=4) were given saline vehicle solution only. The experimental protocol is depicted in Figure 1A, where ΔV is change in tumour volume and R is tumour growth rate. It includes a pre-treatment phase where tumour growth rates were measured; two treatment stages (treatment 1 and 2) where the efficacy of anticancer agents were determined; and two periods with no treatment (off-treatment 1 and 2) where tumour re-growth was quantified. Day 1 was assigned as the first day the mice were imaged and the mice were imaged three times during the 21-day pre-treatment period, and at intervals between 8 to 15 days thereafter. The mice were treated daily by oral gavage during two 28-day treatment sessions. The first treatment session (treatment 1) ran from day 23 to 50, followed by a 21-day period without any treatment (off-treatment 1). The second 28 day treatment session (treatment 2) was from day 72 to 99, followed by a period without any treatment (off-treatment 2), ending on day 119. At the end of the study, the mice were sacrificed and tumours were extracted and fixed in 10% formalin. [2] |
| ADME/Pharmacokinetics |
Pharmacokinetics[10]
Pharmacokinetic parameters of pictilisib were estimated for all dose cohorts and are summarized in Table 3 and Supplementary Table 1. Under fasting conditions, pictilisib was rapidly absorbed after oral administration (median Tmax of 2 hours [range 0.5-8]); this was independent of dose and was unchanged after multiple doses. Terminal plasma elimination half-life (T1/2) on day 1 ranged between 13.1 and 24.1 hours. Dose-proportional increases in exposure (Cmax and AUC0-24) was observed across the dose levels studied (Figure 1). Similar pharmacokinetic characteristics were seen on day 15. The accumulation index (AUCDay15/AUCDay1) ranged from 1.2 to 2.2, suggesting modest accumulation following multiple doses. The absorption, metabolism and excretion of pictilisib, a selective small molecule inhibitor of class 1 A phosphoinositide 3-kinase (PI3K), was characterized following a single oral administration of [14C]pictilisib in rats, dogs and humans at the target doses of 30 mg/kg, 5 mg/kg and 60 mg, respectively.Pictilisib was rapidly absorbed with Tmax less than 2 h across species. In systemic circulation, pictilisib represented the predominant total radioactivity greater than 86.6% in all species.Total pictilisib and related radioactivity was recovered from urine and faeces in rats, dogs, and human at 98%, 80% and 95%, respectively, with less than 2% excreted in urine and the rest excreted into faeces.In rat and dog, more than 40% of drug-related radioactivity was excreted into the bile suggesting biliary excretion was the major route of excretion. Unchanged pictilisib was a minor component in rat and dog bile. The major metabolite in bile was O-glucuronide of oxidation on indazole moiety (M20, 21% of the dose) in rats and an oxidative piperazinyl ring-opened metabolite M7 (10.8% of the dose) in dogs.Oxidative glutathione (GSH) conjugates (M18, M19) were novel metabolites detected in rat bile, suggesting the potential generation of reactive intermediates from pictilisib. The structure of M18 was further confirmed by NMR to be a N-hydroxylated and GSH conjugated metabolite on the moiety of the indazole ring. Xenobiotica . 2021 Jul;51(7):796-810. https://pubmed.ncbi.nlm.nih.gov/33938357/ |
| Toxicity/Toxicokinetics |
Safety and tolerability [10]
Pictilisib was well-tolerated up to 330mg (21/28 schedule); most adverse events were mild to moderate in severity with no treatment-related deaths (Table 2). At the assessed dose levels, there did not appear to be a significant difference in the toxicity profile between the 21/28 and 28/28 schedules. Treatment-related adverse events that occurred in ≥10% of patients included: nausea, diarrhea, vomiting, fatigue, dysgeusia, decreased appetite and rash. In addition to the 2 DLTs of grade 3 rash at the 450mg dose level, the third patient at this dose level experienced grade 2 rash; nonetheless, this patient received 8 months of pictilisib with concomitant use of oral antihistamines and skin emollients. Of 10 patients treated with 330mg once-daily(28/28 schedule), grade 1 or 2 rash was observed in 2 patients, and grade 3 rash (occurring after the DLT-defining window) in 2 patients; these similarly resolved with the introduction of drug holidays and supportive medications including emollients and corticosteroids. Other clinically-relevant drug-related adverse events ≥grade 3 were grade 4 hyperglycemia (n=1, 130mg) and grade 3 pneumonitis (n=1, 340mg). The grade 4 hyperglycemia was transient, unaccompanied by clinically significant symptoms, signs or acidosis, and occurred in a patient with cholangiocarcinoma and previous pancreatico-duodenectomy who started the use of low-dose prednisolone 2 days prior to the event. Grade 3 pneumonitis was observed at the end of cycle 1 in a breast cancer patient previously treated with chest radiotherapy who developed grade 1 dyspnea, reduced DLCO and a ground glass appearance on HRCT; these resolved following 2 weeks of drug interruption and concomitant use of prednisolone. When pictilisib was reintroduced at 240mg, the dyspnea and HRCT changes recurred; these subsequently resolved following permanent discontinuation of pictilisib due to disease progression. DLTs and MTD [10] The MTD was exceeded at 450mg once-daily (21/28 schedule) with a DLT of grade 3 rash in 2 patients. This was a maculopapular rash covering 70-80% of the body surface area that presented approximately 2 weeks after commencement of daily pictilisib dosing and resolved spontaneously 2 weeks after treatment discontinuation. At 330mg once-daily (21/28 schedule), the grade 3 maculopapular rash observed in 1 of 7 patients had a similar temporal pattern of onset and resolution; this was also declared as a DLT. On the 28/28 schedule, no DLT was observed. |
| References |
|
| Additional Infomation |
Pictrelisib is a sulfonamide composed of indazole, morpholine, and methylsulfonyl-substituted piperazine rings bound to a thienopyrimidine ring. It has a role as an EC 2.7.1.137 (phosphatidylinositol 3-kinase) inhibitor. It is a sulfonamide, a member of piperazines, a member of morpholines, a member of indazoles and a thienopyrimidine.
Pictilisib has been used in trials studying the treatment of Solid Cancers, Breast Cancer, Advanced Solid Tumours, Metastatic Breast Cancer, and Non-Hodgkin's Lymphoma, Solid Cancers, among others. Pictilisib is a small molecule inhibitor of class I phosphatidylinositol 3 kinase (PI3K), with potential antineoplastic activity. Upon administration, pictilisib selectively binds to PI3K in an ATP-competitive manner, inhibiting the production of the secondary messenger phosphatidylinositol-3,4,5-trisphosphate (PIP3) and activation of the PI3K/Akt signaling pathway. This may result in inhibition of tumor cell growth, motility and survival in susceptible tumor cell populations. Activation of the PI3K/Akt signaling pathway is frequently associated with tumorigenesis; dysregulated PI3K/Akt signaling may contribute to tumor resistance to a variety of antineoplastic agents. Purpose: Docetaxel is a front-line standard-of-care chemotherapeutic drug for the treatment of breast cancer. Phosphoinositide 3-kinases (PI3K) are lipid kinases that regulate breast tumor cell growth, migration, and survival. The current study was intended to determine whether GDC-0941, an orally bioavailable class I selective PI3K inhibitor, enhances the antitumor activity of docetaxel in human breast cancer models in vitro and in vivo. Experimental design: A panel of 25 breast tumor cell lines representing HER2+, luminal, and basal subtypes were treated with GDC-0941, docetaxel, or the combination of both drugs and assayed for cellular viability, modulation of PI3K pathway markers, and apoptosis induction. Drug combination effects on cellular viability were also assessed in nontransformed MCF10A human mammary epithelial cells. Human xenografts of breast cancer cell lines and patient-derived tumors were used to assess efficacy of GDC-0941 and docetaxel in vivo. Results: Combination of GDC-0941 and docetaxel decreased the cellular viability of breast tumor cell lines in vitro but to variable degrees of drug synergy. Compared with nontransformed MCF10A cells, the addition of both drugs resulted in stronger synergistic effects in a subset of tumor cell lines that were not predicted by breast cancer subtype. In xenograft models, GDC-0941 enhanced the antitumor activity of docetaxel with maximum combination efficacy observed within 1 hour of administering both drugs. GDC-0941 increased the rate of apoptosis in cells arrested in mitosis upon cotreatment with docetaxel. Conclusion: GDC-0941 augments the efficacy of docetaxel by increasing drug-induced apoptosis in breast cancer models.[1] Aim: To assess the efficacy of multiple treatment of phosphatidylinositol-3-kinase (PI3K) inhibitor on autochthonous tumours in phosphatase and tensin homologue (Pten)-deficient genetically engineered mouse cancer models using a longitudinal magnetic resonance imaging (MRI) protocol. Materials and methods: Using 3D MRI, B-cell follicular lymphoma growth was quantified in a Pten(+/-)Lkb1(+/hypo) mouse line, before, during and after repeated treatments with a PI3K inhibitor GDC-0941 (75 mg/kg). Results: Mean pre-treatment linear tumour growth rate was 16.5±12.8 mm(3)/week. Repeated 28-day GDC-0941 administration, with 21 days 'off-treatment', induced average tumour regression of 41±7%. Upon cessation of the second treatment (which was not permanently cytocidal), tumours re-grew with an average linear growth rate of 40.1±15.5 mm(3)/week. There was no evidence of chemoresistance. Conclusion: This protocol can accommodate complex dosing schedules, as well as combine different cancer therapies. It reduces biological variability problems and resulted in a 10-fold reduction in mouse numbers compared with terminal assessment methods. It is ideal for preclinical efficacy studies and for phenotyping molecularly characterized mouse models when investigating gene function.[2] Lung cancer is a malignant disease with poor outcome, which has led to a search for new therapeutics. The PI3K/Akt/mTOR and Ras/raf/Erk pathways are key regulators of tumor growth and survival. In the present study, their roles were evaluated by MTT assay, flow cytometry and Western blotting in lung cancer cells. We found that a high efficacy of antitumor activity was shown with GDC-0941 treatment in two gefitinib-resistant non-small cell lung cancer (NSCLC) cell lines, A549 and H460. In addition, H460 cells with activating mutations of PIK3CA were relatively more sensitive to GDC-0941 than A549 cells with wild-type PIK3CA. Furthermore, GDC-0941 was highly efficacious in combination with U0126 in inducing cell growth inhibition, G0-G1 arrest and cell apoptosis. These antitumor activities of combined treatment may be attributed to the alterations of G0-G1 phase regulators, apoptosis-related proteins and eukaryotic translation initiation factor 4B (eIF4B), induced by concomitant blockade of the PI3K/Akt/mTOR and Ras/raf/Erk pathways. In conclusion, this study suggests that multi‑targeted intervention is the most effective treatment for tumors. Additionally, the blockade of PI3K, mTOR and Erk with GDC-0941 and MEK inhibitors shows promise for treating gefitinib-resistant NSCLC.[3] |
| Molecular Formula |
C23H27N7O3S2
|
|---|---|
| Molecular Weight |
513.6356
|
| Exact Mass |
513.161
|
| Elemental Analysis |
C, 53.78; H, 5.30; N, 19.09; O, 9.34; S, 12.49
|
| CAS # |
957054-30-7
|
| Related CAS # |
Pictilisib dimethanesulfonate;957054-33-0
|
| PubChem CID |
17755052
|
| Appearance |
White to off-white solid powder
|
| Density |
1.5±0.1 g/cm3
|
| Boiling Point |
687.7±65.0 °C at 760 mmHg
|
| Flash Point |
369.7±34.3 °C
|
| Vapour Pressure |
0.0±2.1 mmHg at 25°C
|
| Index of Refraction |
1.753
|
| LogP |
2.04
|
| Hydrogen Bond Donor Count |
1
|
| Hydrogen Bond Acceptor Count |
10
|
| Rotatable Bond Count |
5
|
| Heavy Atom Count |
35
|
| Complexity |
832
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
O=S(C)(N1CCN(CC2=CC3N=C(C4C5=C(NN=C5)C=CC=4)N=C(C=3S2)N2CCOCC2)CC1)=O
|
| InChi Key |
LHNIIDJUOCFXAP-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27)
|
| Chemical Name |
4-(2-(1H-indazol-4-yl)-6-((4-(methylsulfonyl)piperazin-1-yl)methyl)thieno[3,2-d]pyrimidin-4-yl)morpholine
|
| Synonyms |
Pictrelisib; Pictilisib; RG7321; 957054-30-7; GDC-0941; PICTILISIB; Pictrelisib; 4-(2-(1H-Indazol-4-yl)-6-((4-(methylsulfonyl)piperazin-1-yl)methyl)thieno[3,2-d]pyrimidin-4-yl)morpholine; GDC 0941; 2-(1H-Indazol-4-yl)-6-(4-methanesulfonylpiperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine; RG-7321; RG 7321; GDC-0941; GDC 0941; GDC0941; GNE0941; GNE-0941; GNE 0941
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
DMSO: ~44 mg/mL (85.66 mM)
Water: <1 mg/mL (slightly soluble or insoluble) Ethanol: <1 mg/mL (slightly soluble or insoluble) |
|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (4.87 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.5 mg/mL (4.87 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: 2.5 mg/mL (4.87 mM) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Solubility in Formulation 4: 2%DMSO+30%PEG 300+5%Tween 80+ddH2O: 5mg/mL Solubility in Formulation 5: 5 mg/mL (9.73 mM) in 0.5% MC 0.5% Tween-80 (add these co-solvents sequentially from left to right, and one by one), Suspened solution; with ultrasonication. |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9469 mL | 9.7344 mL | 19.4689 mL | |
| 5 mM | 0.3894 mL | 1.9469 mL | 3.8938 mL | |
| 10 mM | 0.1947 mL | 0.9734 mL | 1.9469 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Status | Interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00960960 | Completed | Drug: Bevacizumab Drug: Pictilisib |
Breast Cancer | Genentech, Inc. | August 2009 | Phase 1 |
| NCT01493843 | Completed | Drug: pictilisib Drug: Placebo |
Non-Small Cell Lung Cancer | Genentech, Inc. | January 20, 2012 | Phase 2 |
|
 |
 |