
| Size | Price | Stock | Qty |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g | |||
| Other Sizes |
Purity: ≥98%
Erlotinib HCl (formerly OSI-744, OSI744; trade name: Tarceva), the hydrochloride salt of erlotinib, is an EGFR (epidermal growth factor receptor) inhibitor with antitumor activity. In cell-free experiments, it inhibits EGFR with an IC50 of 2 nM, and when compared to human c-Src or v-Abl, it is >1000 times more sensitive to inhibit EGFR. The FDA and other nations have authorized erlotinib, a quinazoline derivative, for the treatment of pancreatic cancer, non-small cell lung cancer (NSCLC), and various other cancer types.
| Targets |
EGFR (IC50 = 2 nM)
|
|---|---|
| ln Vitro |
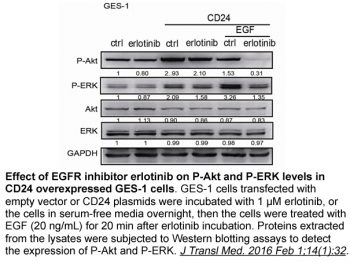
Erlotinib HCl potently inhibits EGFR activation in intact cells, such as MDA MB-468 human breast cancer cells, DiFi human colon cancer cells, and HNS human head and neck tumor cells (IC50 20 nM). DiFi human colon cancer cells undergo apoptosis when exposed to 1 μM erlotinib HCl.[1] With an IC50 ranging from 29 nM to >20 μM, erlotinib inhibits the growth of a panel of NSCLC cell lines, including A549, H322, H3255, H358 H661, H1650, H1975, H1299, and H596.[2] Erlotinib HCl (2 μM) strongly suppresses the proliferation of BxPC-3 and AsPC-1 pancreatic cells.[3] When combined with gemcitabine, the effects of erlotinib HCl are thought to be additive in pancreatic cancer cells that have mutated KRAS. EGFR phosphorylation at the Y845 (Src-dependent phosphorylation) and Y1068 (auto-phosphorylation) sites is inhibited by ten micromolar of erlotinib HCl.[4] When combined with erlotinib HCl, rapamycin-stimulated Akt activity may be down-regulated, and this has a synergistic effect on inhibiting cell growth. [5]
Erlotinib (CP-358774) is also a potent inhibitor, with an IC50 of 1 nM, of the EGFR's recombinant intracellular (kinase) domain. Erlotinib severely inhibits the proliferation of DiFi cells, with an IC50 of 100 nM for an 8-day proliferation assay[1]. When B-DIM and Erlotinib (2 μM) are combined, BxPC-3 cell colony formation is significantly inhibited as opposed to when either agent is used alone. Only in BxPC-3 cells does the combination of B-DIM and Erlotinib (2 μM) significantly induce apoptosis when compared to the apoptotic effect of either agent alone[2]. The epidermal growth factor receptor (EGFR) is overexpressed in a significant percentage of carcinomas and contributes to the malignant phenotype. CP-358,774 is a directly acting inhibitor of human EGFR tyrosine kinase with an IC50 of 2 nM and reduces EGFR autophosphorylation in intact tumor cells with an IC50 of 20 nM. This inhibition is selective for EGFR tyrosine kinase relative to other tyrosine kinases we have examined, both in assays of isolated kinases and whole cells. At doses of 100 mg/kg, CP-358,774 completely prevents EGF-induced autophosphorylation of EGFR in human HN5 tumors growing as xenografts in athymic mice and of the hepatic EGFR of the treated mice. CP-358,774 inhibits the proliferation of DiFi human colon tumor cells at submicromolar concentrations in cell culture and blocks cell cycle progression at the G1 phase. This inhibitor produces a marked accumulation of retinoblastoma protein in its underphosphorylated form and accumulation of p27KIP1 in DiFi cells, which may contribute to the cell cycle block. Inhibition of the EGFR also triggers apoptosis in these cells as determined by formation of DNA fragments and other criteria. These results indicate that CP-358,774 has potential for the treatment of tumors that are dependent on the EGFR pathway for proliferation or survival. [1] Effects of B-DIM and Erlotinib on the Viability of Pancreatic Cancer Cells [2] It is important to note that, during our pilot studies, as indicated in Materials and Methods, different concentrations of B-DIM and erlotinib were used and are presented in Table 1. Moreover, after analyzing the basal level of expression of EGFR, NF-κB, and COX-2, we chose two cell lines having constitutively activated levels of NF-κB, EGFR, and COX-2 expression (BxPC-3) compared with lower level of NF-κB, EGFR, and COX-2 expression (MIAPaCa). Our results prompted us to select the subsequent concentration of B-DIM and erlotinib as presented below. Cell viability of BxPC-3 and MIAPaCa pancreatic cancer cells treated with B-DIM (20 µmol/L), erlotinib (2 µmol/L), and the combination was determined by the MTT assay, and the data are presented in Fig. 1A and B. Significant inhibition of cell viability was seen in BxPC-3 cells treated with either agent, and this was further enhanced by the combination treatment (P = 0.0001). In addition, we have also tested the effects of treatment on cell viability by clonogenic assay as shown below. Similar treatments of MIAPaCa cells resulted in a significant inhibition of viable cells with B-DIM alone but not when exposed to similar concentrations of B-DIM and erlotinib for the same time, and the effect was not enhanced by the combination treatment (P = 0.0890). The insensitivity of MIAPaCa cells to erlotinib is consistent with a recently published report Inhibition of Cell Growth/Survival by Clonogenic Assay [2] To determine the effect of B-DIM and Erlotinib on cell growth, cells were treated with each of the single agents or their combination and assessed for cell viability by clonogenic assay. The combination of B-DIM and erlotinib resulted in a significant inhibition of colony formation in BxPC-3 cells when compared with either agent alone (Fig. 2A and B). Similar treatment of MIAPaCa cells (Fig. 2C) showed inhibition of colony formation with B-DIM alone and also the combination, but the effect was not enhanced with the combination treatment as was seen in BxPC-3 cells (Fig. 2A and B). These results were similar to those obtained from the soft-agar assay. Overall, the results from clonogenic assay was consistent with the MTT data as shown in Fig. 1A and B, suggesting that B-DIM had a differential effect between BxPC-3 and MIAPaCa pancreatic cancer cells. The mechanisms of such differences were further investigated, and the results are presented in the following sections, but first we have determined the effects of B-DIM, erlotinib, and the combination on apoptotic cell death. Induction of Apoptosis by Erlotinib, B-DIM, and the Combination [2] The underlying mechanism on the inhibition of cell viability was further studied by determining the apoptotic effects of different treatments using the Cell Death Detection ELISA. The combination of B-DIM and erlotinib resulted in a significant induction of apoptosis only in BxPC-3 cells when compared with the apoptotic effect of either agent alone (Fig. 1C). Similar treatment of MIAPaCa cells showed no induction of apoptosis with the combination (Fig. 1D). These results are consistent with cell viability assay by MTT. Subsequently, we sought to find further evidence of apoptosis as presented below. B-DIM Enhances Apoptosis Signaling by Erlotinib [2] PARP cleavage was determined in BxPC-3 and MIAPaCa cells that were treated with B-DIM (20 µmol/L), erlotinib (2 µmol/L), and the combination (Fig. 3). We found significant amount of PARP (116 kDa) protein cleavage product (85 kDa fragment) after 72-h treatment only in BxPC-3 cells (Fig. 3). In contrast, MIAPaCa cells treated similarly showed only a small cleavage of PARP with B-DIM alone and also in combination but not with erlotinib alone. The induction of apoptosis could be partly due to inactivation of important survival genes; hence, we investigated whether B-DIM, erlotinib, and their combination could affect key survival proteins. Effect of B-DIM on Molecules Related to Apoptosis [2] BxPC-3 and MIAPaCa cells were used to evaluate the effects of B-DIM and/or Erlotinib on the expression of survivin, Bcl-2, Bcl-xL, and c-IAP1/2. Expression of Bcl-2, Bcl-xL, survivin, and c-IAP1/2 proteins was significantly reduced in cells treated with the combination when compared with either agent alone (Fig. 3). There was no influence on antiapoptotic proteins in MIAPaCa cells treated with either agent alone or the combination. These results suggest that B-DIM, erlotinib, and the combination down-regulate key survival proteins and in turn induced apoptotic cell death in BxPC-3 cells but not in MIAPaCa cells. To further determine the molecular mechanism by which B-DIM sensitized BxPC-3 cells to erlotinib-induced inhibition of cell viability and induction of apoptosis, we investigated the role of EGFR and its downstream signaling pathways. Effect of B-DIM on the Expression of EGFR Protein [2] The expression of EGFR was determined by immunoblotting. No baseline expression of EGFR was found in the MIAPaCa cells. EGFR-expressing BxPC-3 cells showed a significant reduction in the expression of EGFR and phosphorylated EGFR levels when exposed to erlotinib plus B-DIM compared with either agent alone (Fig. 3). It is known that the activation of EGFR could in turn regulate an important transcription factor, NF-κB, which is a known regulator of several survival genes such as survivin, c-IAP1/2, Bcl-2, and Bcl-xL. Because we found a greater degree of down-regulation of survivin, c-IAP1/2, Bcl-2, and Bcl-xL in BxPC-3 cells treated with B-DIM and erlotinib compared with either agent alone, and because these genes are transcriptionally regulated by NF-κB, we investigated the effect of each treatment on the DNA-binding activity of NF-κB. B-DIM Inhibits NF-κBDNA-Binding Activity [2] The activation of NF-κB, a nuclear transcriptional factor, was assessed in B-DIM-treated and Erlotinib-treated cells. There was a significant inhibition of NF-κB activation in BxPC-3 cells exposed to both erlotinib and B-DIM compared with erlotinib alone (Fig. 4A). No such inhibition was shown in the MIAPaCa cells (Fig. 4B). These results suggest that the combination of B-DIM and erlotinib causes greater inhibition of cell growth, induction of apoptosis, inhibition of survival factors, inhibition of EGFR, and inactivation of NF-κB. Because NF-κB plays important roles in the regulation of prosurvival and antiapoptotic processes, we tested whether overexpression of NF-κB by p65 cDNA transfection could abrogate B-DIM-induced and erlotinib-induced apoptotic processes. Moreover, it is known that NF-κB transcriptionally regulates COX-2, which produces PGE2 and in turn induces cell viability. Thus, we tested whether celecoxib, erlotinib, or B-DIM alone could influence the activity of B-DIM and erlotinib in p65 cDNA transfected cells. Erlotinib, B-DIM, and Celecoxib Abrogated Activation of NF-κBActivity Stimulated by p65 cDNA Transfection [2] Cytoplasmic and nuclear proteins from BxPC-3 and MIAPaCa cells transfected with p65 cDNA and then treated with erlotinib (2 µmol/L), B-DIM (20 µmol/L), or celecoxib (5 µmol/L) or left untreated for 48 h were subjected to analysis for NF-κB activity as measured by Western blot analysis and EMSA. The results showed that erlotinib, B-DIM, and celecoxib inhibited the p65 protein and NF-κB DNA-binding activity more in BxPC-3 cells compared with untreated cells (Fig. 5A and B) and very little effect was seen in MIAPaCa cells. Importantly, NF-κB p65 cDNA transfection enhanced the NF-κB p65 protein and DNA-binding activity only in BxPC-3 cells to a significant level as shown in Fig. 5A and B. On the other hand, no such changes were observed in the MIAPaCa cells. Because the activation of NF-κB induces COX-2 expression leading to the production of PGE2 that is released into the culture medium, we measured the levels of PGE2 in untransfected and transfected cells treated with erlotinib, B-DIM, and the COX-2 inhibitor celecoxib. Inhibition of PGE2 Synthesis in p65 cDNA-Transfected Cells [2] We measured the levels of PGE2 in the conditioned medium collected from both BxPC-3 and MIAPaCa cells as an indicator of COX-2 activity. We found a high level of PGE2 secretion by BxPC-3 cells, whereas MIAPaCa cells showed very low levels of PGE2, which is consistent with its low constitutive expression of COX-2. BxPC-3 and MIAPaCa cells were transfected with p65 cDNA followed by treatment with Erlotinib (10 nmol/L), B-DIM (1 µmol/L), or celecoxib (1 nmol/L) to analyze the levels of PGE2 released into the culture medium (Fig. 5C). No change in PGE2 level was noted when cells were treated with erlotinib alone (P = 0.084). However, a significant reduction in PGE2 level was observed in BxPC-3 cells treated with B-DIM (P = 0.006) and celecoxib (P = 0.005). There was a substantial increase in the PGE2 level in p65 cDNA-transfected BxPC-3 cells compared with untransfected cells (P = 0.009), suggesting that NF-κB could induce COX-2 expression. However, there was no change in PGE2 level in MIAPaCa cells with any of the agents. Collectively, these results suggest that the production of PGE2 is mediated through the NF-κB and COX-2 pathway and that celecoxib could down-regulate both NF-κB and COX-2. These results were subsequently correlated with the degree of apoptosis (Fig. 5D) as presented below. Apoptosis through the Inactivation of NF-κB in p65 cDNA-Transfected Cells [1] p65 cDNA was transfected into BxPC-3 and MIAPaCa cells and then treated with Erlotinib (2 µmol/L), B-DIM (20 µmol/L), or celecoxib (5 µmol/L) for 48 h (Fig. 5D). The degree of apoptosis in p65 cDNA-transfected BxPC-3 cells treated with erlotinib (P = 0.034) was much less compared with untransfected cells treated with erlotinib (P = 0.007). Similar results were observed with both B-DIM and celecoxib treatment in BxPC-3 cells. However, in MIAPaCa cells, no such degree of apoptosis was observed. These results suggest that activation of NF-κB by p65 cDNA transfection could abrogate the apoptosis inducing effect of erlotinib, B-DIM, and celecoxib. |
| ln Vivo |
Erlotinib HCl fully inhibits EGF-induced autophosphorylation of EGFR in human HN5 tumors growing as xenografts in athymic mice, as well as of the treated mice's hepatic EGFR, at doses of 100 mg/kg.[1] H460a and A549 tumor models are inhibited by erlotinib HCl (100 mg/Kg) at 71 and 93% inhibition rates, respectively.[5]
In comparison to the untreated control, the combination of B-DIM and Erlotinib (50 mg/kg, i.p.) treatment significantly (P <0.01) reduces tumor weight under experimental conditions[2]. In comparison to the CP+vehicle (V) rats, erlotinib (20 mg/kg, p.o.) significantly attenuates the body weight (BW) loss induced by Cisplatin (CP) (P<0.05). Treatment with erlotinib considerably enhances renal function in CP-N (normal control group, NC) rats. Compared to the CP+V rats, the CP+Erlotinib (E) rats exhibit a significant increase in urine volume (UV) (P<0.05) and Cr clearance (Ccr) (P<0.05), as well as a significant decrease in serum creatinine (s-Cr) (P<0.05), blood urea nitrogen (BUN) (P<0.05), and urinary N-acetyl-β-D-glucosaminidase (NAG) index (P<0.05). B-DIM Augments In vivo Therapeutic Effect of Erlotinib on Primary Tumor [2] Potential therapeutic utility of B-DIM and erlotinib combination in SCID mice bearing orthotopically implanted BxPC-3 pancreatic tumor cells was investigated. A dose of 3.5 mg/d B-DIM per mouse was selected for p.o. administration, whereas erlotinib dose (50 mg/kg body weight i.p.) was based on previously published reports as shown in Fig. 6A. A total of 28 mice were divided into four groups. To ascertain the efficacy of a single-agent treatment compared with combinations, we determined the mean pancreas weight in all treated groups. Under our experimental conditions, administration of B-DIM by gavage treatment and erlotinib alone caused 20% and 35% reduction in tumor weight, respectively, compared with control tumors (Fig. 6C). However, under the experimental conditions, the combination of B-DIM and erlotinib treatment showed significant decrease (P < 0.01) in tumor weight compared with untreated control, B-DIM alone, or erlotinib alone treatment group. These results showed, for the first time, the efficacy of combination of B-DIM and erlotinib in the inhibition of pancreatic tumor growth in an orthotopic model. B-DIM Inhibits NF-κBDNA-Binding Activity In vivo [2] The activation of NF-κB was assessed in B-DIM-treated and Erlotinib-treated tumor tissues. The results show that NF-κB was down-regulated by B-DIM and erlotinib (Fig. 6B). Fig. 6B (bottom) represents results from all seven mice. These in vivo results were similar to our in vitro findings, suggesting that the inactivation of NF-κB is, at least, one of the molecular mechanisms by which B-DIM potentiates erlotinib-induced antitumor activity in our experimental animal model. The effects of blocking the epidermal growth factor receptor (EGFR) in acute kidney injury (AKI) are controversial. Here we investigated the renoprotective effect of Erlotinib, a selective tyrosine kinase inhibitor that can block EGFR activity, on cisplatin (CP)-induced AKI. Groups of animals were given either Erlotinib or vehicle from one day before up to Day 3 following induction of CP-nephrotoxicity (CP-N). In addition, we analyzed the effects of erlotinib on signaling pathways involved in CP-N by using human renal proximal tubular cells (HK-2). Compared to controls, rats treated with erlotinib exhibited significant improvement of renal function and attenuation of tubulointerstitial injury, and reduced the number of apoptotic and proliferating cells. Erlotinib-treated rats had a significant reduction of renal cortical mRNA for profibrogenic genes. The Bax/Bcl-2 mRNA and protein ratios were significantly reduced by erlotinib treatment. In vitro, we observed that erlotinib significantly reduced the phosphorylation of MEK1 and Akt, processes that were induced by CP in HK-2. Taken together, these data indicate that erlotinib has renoprotective properties that are likely mediated through decreases in the apoptosis and proliferation of tubular cells, effects that reflect inhibition of downstream signaling pathways of EGFR. These results suggest that erlotinib may be useful for preventing AKI in patients receiving CP chemotherapy[PLoS One. 2014 Nov 12;9(11):e111728.]. |
| Enzyme Assay |
The process of coating 96-well plates involves incubating 100 μL of 0.25 mg/mL PGT in PBS per well for an entire night at 37 °C. Aspiration is used to remove excess PGT, and three washing buffer washes (0.1% Tween 20 in PBS) are performed on the plate. 50 μL of 50 mM HEPES (pH 7.3) containing 0.1 mM sodium orthovanadate, 125 mM sodium chloride, 24 mM magnesium chloride, 20 μM ATP, 1.6 μg/mL EGF, and 15 ng of affinity-purified EGFR from A431 cell membranes is used for the kinase reaction. A final DMSO concentration of 2.5% is achieved by adding erlotinib HCl in DMSO. When ATP is added, phosphorylation begins and continues for eight minutes at room temperature while being constantly shaken.The kinase reaction is terminated by aspiration of the reaction mixture and is washed 4 times with washing buffer. Phosphorylated PGT is measured by 25 minutes of incubation with 50 μL per well HRP-conjugated PY54 antiphosphotyrosine antibody, diluted to 0.2 μg/mL in blocking buffer (3% BSA and 0.05% Tween 20 in PBS). Antibody is removed by aspiration, and the plate is washed 4 times with washing buffer. The colonmetric signal is developed by addition of TMB Microwell Peroxidase Substrate, 50μL per well, and stopped by the addition of 0.09 M sulfuric acid, 50 μL per well. Phosphotyrosine is estimated by measurement of absorbance at 450 nm. In wells without AlP, EGFR, or PGT, the signal for controls is usually between 0.6 and 1.2 absorbance units, with almost no background, and it is proportional to the incubation period of 10 minutes.
|
| Cell Assay |
Seeded in triplicate, exponentially growing cells are subjected for 72 hours to serial dilutions of erlotinib, pemetrexed, or the combination at a constant concentration ratio of 4:1. Cell count and the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay are used to measure cell viability. The percentage of drug-treated control cells that survive compared to PBS-treated control cells (which are thought to be 100% viable) is known as growth inhibition. The CalcuSyn software determines the IC50 value, which is the concentration at which a 72-hour exposure to drug(s) results in a 50% inhibition of cell growth when compared to untreated control cells.
In order to assess the survival of cells treated with B-DIM, Erlotinib, or both, 3,000–5,000 BxPC-3 and MIAPaCa cells are plated per well in a 96-well plate and incubated at 37°C for the entire night. Initially, B-DIM (10-50 µM) and Erlotinib (1-5 µM) are tested at a range of concentrations. The concentrations of B-DIM (20 µM) and Erlotinib (2 µM) are selected for each assay based on the preliminary findings. The standard MTT assay is used to measure the effects of B-DIM (20 µM), Erlotinib (2 µM), and the combination on BxPC-3 and MIAPaCa cells. The assay is performed three times after 72 hours. The Tecan microplate fluorometer measures the color intensity at 595 nm. Cells treated with DMSO are given a value of 100% and are regarded as the untreated control. We have performed clonogenic assay in addition to the aforementioned assay to evaluate the effects of treatment[2]. Cell Viability Assay [2] To test the viability of cells treated with B-DIM, Erlotinib, or the combination, BxPC-3 and MIAPaCa cells were plated (3,000–5,000 per well) in a 96-well plate and incubated overnight at 37°C. We initially tested a range of concentrations for both B-DIM (10–50 µmol/L) and erlotinib (1–5 µmol/L). Based on the initial results, the concentration of B-DIM (20 µmol/L) and erlotinib (2 µmol/L) were chosen for all assays. The effects of B-DIM (20 µmol/L), erlotinib (2 µmol/L), and the combination on BxPC-3 and MIAPaCa cells were determined by the standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay after 72 h and was repeated three times. The color intensity was measured by a Tecan microplate fluorometer at 595 nm. DMSO-treated cells were considered to be the untreated control and assigned a value of 100%. In addition to the above assay, we have also done clonogenic assay for assessing the effects of treatment as shown below. Clonogenic Assay [2] To test the survival of cells treated with B-DIM, Erlotinib, or the combination, BxPC-3 and MIAPaCa cells were plated (50,000–100,000 per well) in a six-well plate and incubated overnight at 37°C. After 72-h exposure to 20 µmol/L B-DIM, 2 µmol/L erlotinib, and the combination, the cells were trypsinized, and the viable cells were counted (trypan blue exclusion) and plated in 100 mm Petri dishes in a range of 100 to 1,000 cells to determine the plating efficiency as well as assess the effects of treatment on clonogenic survival. The cells were then incubated for ~10 to 12 days at 37°C in a 5% CO2/5% O2/90% N2 incubator. The colonies were stained with 2% crystal violet and counted. The surviving fraction was normalized to untreated control cells with respect to clonogenic efficiency, which was 83% for both BxPC-3 and MIAPaCa cells. In addition to this assay, cells were also treated similarly and plated in soft-agar (soft-agar colony assay) and incubated at 37°C. The colonies in the soft agar were also counted in all untreated and treated wells after 12 days. Quantification of Apoptosis by ELISA [2] The Cell Death Detection ELISA kit (Roche Applied Science) was used to detect apoptosis in untreated and treated BxPC-3 and MIAPaCa cells. Cells seeded in six-well plates were treated with B-DIM (20 µmol/L), Erlotinib (2 µmol/L), or the combination. The cells were trypsinized and ~10,000 cells were used as described earlier. Tecan microplate fluorometer was used to measure color intensity at 405 nm. The experiment was repeated three times. Protein Extraction and Western Blot Analysis [2] BxPC-3 and MIAPaCa cells treated with B-DIM (20 µmol/L), Erlotinib (2 µmol/L), or the combination for 72 h were used to evaluate the effects of treatment on survivin, Bcl-2, Bcl-xL, EGFR, EGFR-pTyr1173, c-IAP1/2, Src, poly(ADP-ribose) polymerase (PARP), and β-actin expression. The experiment was carried out for a minimum of three times. Cells were harvested as described previously. The samples were loaded on 7% to 12% SDSPAGE for separation and electrophoretically transferred to a nitrocellulose membrane. Each membrane was incubated with monoclonal antibody against survivin, Bcl-2, Bcl-xL, Src, c-IAP1/2, EGFR, EGFR-pTyr1173, PARP, and β-actin. Blots were incubated with secondary antibodies conjugated with peroxidase. The signal intensity was then measured using chemiluminescent detection system. Electrophoretic Mobility Shift Assay for NF-κB Activation [2] To evaluate the effect of B-DIM and Erlotinib on BxPC-3 and MIAPaCa cells, the cells were either untreated or treated with B-DIM (20 µmol/L), erlotinib (2 µmol/L), or the combination with a minimum repeat of experiment at least three times for 72 h. The cells or the minced tumor tissue were homogenized using a Dounce homogenizer in 400 µL ice-cold lysis buffer as described earlier. |
| Animal Protocol |
Mice: Erlotinib (5 mg/kg) is administered p.o. or i.p. to Bcrp1/Mdr1a/1b-/- and WT mice. The selection of i.p. administration is predicated on full bioavailability and optimal drug absorption. Three series of samples are taken from the lateral tail vein tip. Whole blood samples are taken during the first series at 15, min, 0.5, 1.5, 5, and 10 h following injection. The sampling times of the two subsequent series are adjusted to 5 and 15 minutes and 0.5, 1.5, 4, and 8 hours after injection based on the findings of this initial group. Blood samples are collected, centrifuged right away, and the plasma is kept at -20°C until high-performance liquid chromatographic analysis is performed.
Rats: There are male Crl:CD (SD) rats (244-297 g) that are seven weeks old. Erlotinib hydrochloride (10 mg/kg and 20 mg/kg) is given orally to the animals by gavage. Mice: The treatment groups consist of seven randomly assigned female ICR-SCID mice, aged 6-7 weeks: (a) control (no treatment); (b) B-DIM (50 mg/kg body weight) administered intragastrically once daily; (c) Erlotinib (50 mg/kg body weight) administered daily intraperitoneally for 15 days; and (d) B-DIM and Erlotinib administered according to the schedule for individual treatments. After receiving their last dose of medication, all mice are killed on day three, and their body weight is recorded. A portion of the tissue is immediately frozen in liquid nitrogen and kept cold (−70°C) for later use, while the remaining portion is fixed in formalin and prepared for paraffin block processing. The presence of a tumor or tumors in each pancreas is verified by staining a fixed tissue section with H&E. Rats: Male Sprague-Dawley (SD) rats six weeks of age, weighing 180–210 g, are utilized. On day 0, SD rats (n=28) receive an intraperitoneal injection of 7 mg/kg of freshly prepared ciprofloxacin (CP) at a concentration of 1 mg/mL. For the purpose of examining Erlotinib's effects, 28 CP-N rats are split into two groups. Animals in two groups (n = 14) are given daily oral gavages of either Erlotinib (20 mg/kg) (CP+E, n = 14) or vehicle (CP+V, n = 14) from day -1 (24 hours before the CP injection) to day 3. Groups treated with vehicles are given the same amount of saline. At six weeks of age, a normal control group (NC, n = 5) consists of five male SD rats. From the first to the third day, the NC rats receive an equivalent volume of saline orally via gavage. Day 4 (96 hours post-CP injection): rats are anesthetized, and following a cardiac puncture, they are sacrificed by exsanguination. The kidneys and blood are simultaneously extracted. Renal tissue is sectioned and fixed in 2% paraformaldehyde/phosphate-buffered saline (PBS) for later use, or it can be snap-frozen in liquid nitrogen. In order to reduce suffering as much as possible, diethyl ether gas anesthesia is used during all surgical procedures. Mice were randomized into the following treatment groups (n = 7): (a) untreated control; (b) only B-DIM (50 mg/kg body weight), intragastric once every day; (c) Erlotinib (50 mg/kg body weight), everyday i.p. for 15 days; and (d) B-DIM and Erlotinib, following schedule as for individual treatments. All mice were killed on day 3 following last dose of treatment, and their body weight was determined. One part of the tissue was rapidly frozen in liquid nitrogen and stored at −70°C for future use and the other part was fixed in formalin and processed for paraffin block. H&E staining of fixed tissue section was used to confirm the presence of tumor(s) in each pancreas. [2] Cisplatin (CP) was freshly prepared in saline at a concentration of 1 mg ml−1 and then injected intraperitoneally in SD rats (n = 28) at a dose of 7 mg/kg on day 0. The dose of CP was selected based on a previous stud. To investigate the effect of Erlotinib, 28 CP-N rats were divided into two groups. Separate groups (n = 14) each of animals were administered with either Erlotinib (20 mg/kg, Cugai Pharmaceutical/F. Hoffmann-La Roche, Basel, Switzerland) (CP+E, n = 14) or vehicle (CP+V, n = 14) daily by oral gavage from day -1 (24 hours prior to the CP injection) to day 3. Vehicle-treated groups received an equivalent volume of saline. Five male SD rats at the age of 6 weeks were used as a normal control group (NC, n = 5). The NC rats were given an equivalent volume of saline daily by oral gavage from day -1 to day 3. At day 4 (96 hours after CP injection), each rat was anesthetized and sacrificed by exsanguination after the cardiac puncture; blood was collected by cardiac puncture and kidneys were collected (Figure 1). Renal tissue was divided; separate portions were snap-frozen in liquid nitrogen or fixed in 2% paraformaldehyde/phosphate-buffered saline (PBS) for later use. All surgery was performed under diethyl ether gas anesthesia, and all efforts were made to minimize suffering. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
Erlotinib is about 60% absorbed after oral administration and its bioavailability is substantially increased by food to almost 100%. Peak plasma levels occur 4 hours after dosing. The solubility of erlotinib is pH dependent. Solubility decreases pH increases. Smoking also decrease the exposure of erlotinib. Following a 100 mg oral dose, 91% of the dose was recovered in which 83% was in feces (1% of the dose as unchanged parent compound) and 8% in urine (0.3% of the dose as unchanged parent compound). Apparent volume of distribution = 232 L Smokers have a 24% higher rate of erlotinib clearance. Erlotinib is about 60% absorbed after oral administration and its bioavailability is substantially increased by food to almost 100%. Peak plasma levels occur 4 hours after dosing. The solubility of erlotinib is pH dependent. Erlotinib solubility decreases as pH increases. Following absorption, erlotinib is approximately 93% protein bound to plasma albumin and alpha-1 acid glycoprotein. Erlotinib has an apparent volume of distribution of 232 liters. Time to reach steady state plasma concentration /is/ 7 - 8 days. No significant relationships of clearance to covariates of patient age, body weight or gender were observed. Smokers had a 24% higher rate of erlotinib clearance. Following a 100 mg oral dose, 91% of the dose was recovered: 83% in feces (1% of the dose as intact parent) and 8% in urine (0.3% of the dose as intact parent). For more Absorption, Distribution and Excretion (Complete) data for Erlotinib (10 total), please visit the HSDB record page. Metabolism / Metabolites Metabolism occurs in the liver. In vitro assays of cytochrome P450 metabolism showed that erlotinib is metabolized primarily by CYP3A4 and to a lesser extent by CYP1A2, and the extrahepatic isoform CYP1A1. Metabolism and excretion of erlotinib, an orally active inhibitor of epidermal growth factor receptor tyrosine kinase, were studied in healthy male volunteers after a single oral dose of (14)C-erlotinib hydrochloride (100-mg free base equivalent, approximately 91 microCi/subject)... In plasma, unchanged erlotinib represented the major circulating component, with the pharmacologically active metabolite M14 accounting for approximately 5% of the total circulating radioactivity. Three major biotransformation pathways of erlotinib are O-demethylation of the side chains followed by oxidation to a carboxylic acid, M11 (29.4% of dose); oxidation of the acetylene moiety to a carboxylic acid, M6 (21.0%); and hydroxylation of the aromatic ring to M16 (9.6%). In addition, O-demethylation of M6 to M2, O-demethylation of the side chains to M13 and M14, and conjugation of the oxidative metabolites with glucuronic acid (M3, M8, and M18) and sulfuric acid (M9) play a minor role in the metabolism of erlotinib. The identified metabolites accounted for >90% of the total radioactivity recovered in urine and feces. The metabolites observed in humans were similar to those found in the toxicity species, rats and dogs. Erlotinib has known human metabolites that include Erlotinib M14. Biological Half-Life Median half-life of 36.2 hours. A population pharmacokinetic analysis in 591 patients receiving the single-agent erlotinib hydrochloride 2nd/3rd line regimen showed a median half-life of 36.2 hours. |
| Toxicity/Toxicokinetics |
Hepatotoxicity
Elevations in serum aminotransferase levels are common during erlotinib therapy of pancreatic and lung cancers, and values above 5 times the upper limit of normal occur in at least 10% of patients. Similar rates of ALT elevations, however, can occur with comparable antineoplastic regimens. The abnormalities are usually asymptomatic and self-limited, but may require dose adjustment or discontinuation (Case 1). In addition, there have been rare reports of clinically apparent liver injury attributed to erlotinib therapy. The time to onset is typically within days or weeks of starting therapy, and the liver injury can be severe, there being at least a dozen fatal instances reported in the literature. The onset of injury can be abrupt and the pattern of serum enzyme elevations is usually hepatocellular (Case 2). Immunoallergic features (rash, fever and eosinophilia) are not common and autoantibody formation has not been reported. Routine monitoring of liver tests during therapy is recommended. The rate of clinically significant liver injury and hepatic failure is increased in patients with preexisting cirrhosis or hepatic impairment due to liver tumor burden. Likelihood score: B (likely but uncommon cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of erlotinib during breastfeeding. Because erlotinib is 93% bound to plasma proteins, the amount in milk is likely to be low. However, its half-life is about 36 hours and it might accumulate in the infant. It is also given in combination with gemcitabine for pancreatic cancer, which may increase the risk to the infant. The manufacturer recommends that breastfeeding be discontinued during erlotinib therapy and for 2 weeks after the final dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding 93% protein bound to albumin and alpha-1 acid glycoprotein (AAG) |
| References | |
| Additional Infomation |
Erlotinib Hydrochloride is the hydrochloride salt of a quinazoline derivative with antineoplastic properties. Competing with adenosine triphosphate, erlotinib reversibly binds to the intracellular catalytic domain of epidermal growth factor receptor (EGFR) tyrosine kinase, thereby reversibly inhibiting EGFR phosphorylation and blocking the signal transduction events and tumorigenic effects associated with EGFR activation.
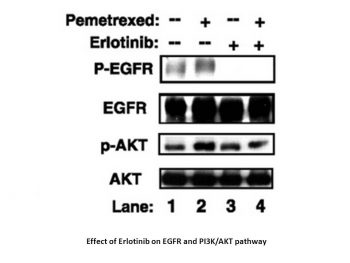
A quinazoline derivative and ANTINEOPLASTIC AGENT that functions as a PROTEIN KINASE INHIBITOR for EGFR associated tyrosine kinase. It is used in the treatment of NON-SMALL CELL LUNG CANCER. See also: Erlotinib (has active moiety). Drug Indication Non-small cell lung cancer (NSCLC)Tarceva is also indicated for switch maintenance treatment in patients with locally advanced or metastatic non-small cell lung cancer with EGFR activating mutations and stable disease after first-line chemotherapy. Tarceva is also indicated for the treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen. In patients with tumours without EGFR activating mutations, Tarceva is indicated when other treatment options are not considered suitable. When prescribing Tarceva, factors associated with prolonged survival should be taken into account. No survival benefit or other clinically relevant effects of the treatment have been demonstrated in patients with Epidermal Growth Factor Receptor (EGFR)-IHC - negative tumours. Pancreatic cancer Tarceva in combination with gemcitabine is indicated for the treatment of patients with metastatic pancreatic cancer . When prescribing Tarceva, factors associated with prolonged survival should be taken into account. The epidermal growth factor receptor (EGFR) is overexpressed in a significant percentage of carcinomas and contributes to the malignant phenotype. CP-358,774 is a directly acting inhibitor of human EGFR tyrosine kinase with an IC50 of 2 nM and reduces EGFR autophosphorylation in intact tumor cells with an IC50 of 20 nM. This inhibition is selective for EGFR tyrosine kinase relative to other tyrosine kinases we have examined, both in assays of isolated kinases and whole cells. At doses of 100 mg/kg, CP-358,774 completely prevents EGF-induced autophosphorylation of EGFR in human HN5 tumors growing as xenografts in athymic mice and of the hepatic EGFR of the treated mice. CP-358,774 inhibits the proliferation of DiFi human colon tumor cells at submicromolar concentrations in cell culture and blocks cell cycle progression at the G1 phase. This inhibitor produces a marked accumulation of retinoblastoma protein in its underphosphorylated form and accumulation of p27KIP1 in DiFi cells, which may contribute to the cell cycle block. Inhibition of the EGFR also triggers apoptosis in these cells as determined by formation of DNA fragments and other criteria. These results indicate that CP-358,774 has potential for the treatment of tumors that are dependent on the EGFR pathway for proliferation or survival.[1] Purpose: This study was undertaken to select the optimal combination schedule of erlotinib and pemetrexed for the treatment of relapsed non-small cell lung cancer (NSCLC) using a panel of human NSCLC lines. Experimental design: Human NSCLC cell lines, with variable expression of the known molecular determinants of erlotinib sensitivity, were exposed to pemetrexed and erlotinib using different schedules. Antitumor effect was measured by growth inhibition by cell count and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, cell cycle distribution and apoptosis by flow cytometry, and expression of cell cycle mediators by immunoblots. The cytotoxic interaction between pemetrexed and erlotinib (i.e., synergistic, additive, or antagonistic) was determined by median effect analysis. Results: When cells were exposed to concurrent pemetrexed and erlotinib or sequential pemetrexed followed by erlotinib, cytotoxic synergism was observed in both erlotinib-sensitive and erlotinib-resistant human NSCLC cell lines. This was independent of the mutation status of epidermal growth factor receptor or K-Ras genes. Synergism was associated with a combination of cell cycle effects from both agents. In contrast, exposure of cells to erlotinib followed by pemetrexed was mostly antagonistic in erlotinib-sensitive cells and additive at best in erlotinib-resistant cells. Antagonism was associated with erlotinib-induced G(1)-phase blockade of erlotinib-sensitive cells, which protects cells from pemetrexed cytotoxicity. Pemetrexed induced an epidermal growth factor receptor-mediated activation of the phosphatidylinositol 3-kinase/AKT pathway, which was inhibited by erlotinib and a specific phosphatidylinositol 3-kinase inhibitor, LY294002. Conclusions: The combination of pemetrexed and erlotinib is synergistic in NSCLC in vitro if exposure to erlotinib before pemetrexed is avoided, particularly in tumors that are sensitive to erlotinib. Based on these findings, a randomized phase II study comparing the progression-free survival between an intermittent combination of erlotinib and pemetrexed (experimental arm) and pemetrexed alone (control arm) in patients with relapsing NSCLC has been initiated.[2] Purpose: This study was undertaken to select the optimal combination schedule of erlotinib and pemetrexed for the treatment of relapsed non-small cell lung cancer (NSCLC) using a panel of human NSCLC lines. Experimental design: Human NSCLC cell lines, with variable expression of the known molecular determinants of erlotinib sensitivity, were exposed to pemetrexed and erlotinib using different schedules. Antitumor effect was measured by growth inhibition by cell count and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, cell cycle distribution and apoptosis by flow cytometry, and expression of cell cycle mediators by immunoblots. The cytotoxic interaction between pemetrexed and erlotinib (i.e., synergistic, additive, or antagonistic) was determined by median effect analysis. Results: When cells were exposed to concurrent pemetrexed and erlotinib or sequential pemetrexed followed by erlotinib, cytotoxic synergism was observed in both erlotinib-sensitive and erlotinib-resistant human NSCLC cell lines. This was independent of the mutation status of epidermal growth factor receptor or K-Ras genes. Synergism was associated with a combination of cell cycle effects from both agents. In contrast, exposure of cells to erlotinib followed by pemetrexed was mostly antagonistic in erlotinib-sensitive cells and additive at best in erlotinib-resistant cells. Antagonism was associated with erlotinib-induced G(1)-phase blockade of erlotinib-sensitive cells, which protects cells from pemetrexed cytotoxicity. Pemetrexed induced an epidermal growth factor receptor-mediated activation of the phosphatidylinositol 3-kinase/AKT pathway, which was inhibited by erlotinib and a specific phosphatidylinositol 3-kinase inhibitor, LY294002. Conclusions: The combination of pemetrexed and erlotinib is synergistic in NSCLC in vitro if exposure to erlotinib before pemetrexed is avoided, particularly in tumors that are sensitive to erlotinib. Based on these findings, a randomized phase II study comparing the progression-free survival between an intermittent combination of erlotinib and pemetrexed (experimental arm) and pemetrexed alone (control arm) in patients with relapsing NSCLC has been initiated.[3] The receptor for epidermal growth factor (EGFR) is overexpressed in many cancers. One important signaling pathway regulated by EGFR is the phosphatidylinositol 3'-kinase (PI3K)-phosphoinositide-dependent kinase 1-Akt pathway. Activation of Akt leads to the stimulation of antiapoptotic pathways, promoting cell survival. Akt also regulates the mammalian target of rapamycin (mTOR)-S6K-S6 pathway to control cell growth in response to growth factors and nutrients. Recent reports have shown that the sensitivity of non-small-cell lung cancer cell lines to EGFR inhibitors such as erlotinib (Tarceva, OSI Pharmaceuticals) is dependent on inhibition of the phosphatidylinositol 3'-kinase-phosphoinositide-dependent kinase 1-Akt-mTOR pathway. There can be multiple inputs to this pathway as activity can be regulated by other receptors or upstream mutations. Therefore, inhibiting EGFR alone may not be sufficient for substantial inhibition of all tumor cells, highlighting the need for multipoint intervention. Herein, we sought to determine if rapamycin, an inhibitor of mTOR, could enhance erlotinib sensitivity for cell lines derived from a variety of tissue types (non-small-cell lung, pancreatic, colon, and breast). Erlotinib could inhibit extracellular signal-regulated kinase, Akt, and S6 only in cell lines that were the most sensitive. Rapamycin could fully inhibit S6 in all cell lines, but this was accompanied by activation of Akt phosphorylation. However, combination with erlotinib could down-modulate rapamycin-stimulated Akt activity. Therefore, in select cell lines, inhibition of both S6 and Akt was achieved only with the combination of erlotinib and rapamycin. This produced a synergistic effect on cell growth inhibition, observations that extended in vivo using xenograft models. These results suggest that combining rapamycin with erlotinib might be clinically useful to enhance response to erlotinib.[4] Our objective was the preclinical assessment of the pharmacokinetics, monotherapy and combined antitumor activity of the epidermal growth factor receptor (HER1/EGFR) tyrosine kinase inhibitor erlotinib in athymic nude mice bearing non-small cell lung cancer (NSCLC) xenograft models. Immunohistochemistry determined the HER1/EGFR status of the NSCLC tumor models. Pharmacokinetic studies assessed plasma drug concentrations of erlotinib in tumor- and non-tumor-bearing athymic nude mice. These were followed by maximum tolerated dose (MTD) studies for erlotinib and each chemotherapy. Erlotinib was then assessed alone and in combination with these chemotherapies in the NSCLC xenograft models. Complete necropsies were performed on most of the animals in each study to further assess antitumor or toxic effects. Erlotinib monotherapy dose-dependently inhibited tumor growth in the H460a tumor model, correlating with circulating levels of drug. There was antitumor activity at the MTD with each agent tested in both the H460a and A549 tumor models (erlotinib 100 mg/kg: 71 and 93% tumor growth inhibition; gemcitabine 120 mg/kg: 93 and 75% tumor growth inhibition; cisplatin 6 mg/kg: 81 and 88% tumor growth inhibition). When each compound was given at a fraction of the MTD, tumor growth inhibition was suboptimal. Combinations of gemcitabine or cisplatin with erlotinib were assessed at 25% of the MTD to determine efficacy. In both NSCLC models, doses of gemcitabine (30 mg/kg) or cisplatin (1.5 mg/kg) with erlotinib (25 mg/kg) at 25% of the MTD were well tolerated. For the slow growing A549 tumor, there was significant tumor growth inhibition in the gemcitabine/erlotinib and cisplatin/erlotinib combinations (above 100 and 98%, respectively), with partial regressions. For the faster growing H460a tumor, there was significant but less remarkable tumor growth inhibition in these same combinations (86 and 53% respectively). These results show that in NSCLC xenograft tumors with similar levels of EGFR expression, the antitumor activity of erlotinib is robust both as monotherapy and in combination with chemotherapies.[5] The receptor for epidermal growth factor (EGFR) is overexpressed in many cancers. One important signaling pathway regulated by EGFR is the phosphatidylinositol 3'-kinase (PI3K)-phosphoinositide-dependent kinase 1-Akt pathway. Activation of Akt leads to the stimulation of antiapoptotic pathways, promoting cell survival. Akt also regulates the mammalian target of rapamycin (mTOR)-S6K-S6 pathway to control cell growth in response to growth factors and nutrients. Recent reports have shown that the sensitivity of non-small-cell lung cancer cell lines to EGFR inhibitors such as erlotinib (Tarceva, OSI Pharmaceuticals) is dependent on inhibition of the phosphatidylinositol 3'-kinase-phosphoinositide-dependent kinase 1-Akt-mTOR pathway. There can be multiple inputs to this pathway as activity can be regulated by other receptors or upstream mutations. Therefore, inhibiting EGFR alone may not be sufficient for substantial inhibition of all tumor cells, highlighting the need for multipoint intervention. Herein, we sought to determine if rapamycin, an inhibitor of mTOR, could enhance erlotinib sensitivity for cell lines derived from a variety of tissue types (non-small-cell lung, pancreatic, colon, and breast). Erlotinib could inhibit extracellular signal-regulated kinase, Akt, and S6 only in cell lines that were the most sensitive. Rapamycin could fully inhibit S6 in all cell lines, but this was accompanied by activation of Akt phosphorylation. However, combination with erlotinib could down-modulate rapamycin-stimulated Akt activity. Therefore, in select cell lines, inhibition of both S6 and Akt was achieved only with the combination of erlotinib and rapamycin. This produced a synergistic effect on cell growth inhibition, observations that extended in vivo using xenograft models. These results suggest that combining rapamycin with erlotinib might be clinically useful to enhance response to erlotinib.[6] Background: Epidermal growth factor receptor (EGFR) and mammalian target of rapamycin (mTOR) are crucial targets in cancer therapy. Combined inhibition of both targets yielded synergistic effects in vitro and in vivo in several cancer entities. However, the impact of EGFR and mTOR expression and combined inhibition in neuroendocrine lung tumors other than small-cell lung cancer remains unclear. Material and methods: Expression and activation of EGFR/AKT/mTOR pathway constituents were investigated in typical and atypical bronchial carcinoid (AC) tumors and large-cell neuroendocrine lung carcinomas (LCNEC) by immunohistochemistry in 110 tumor samples, and correlated with clinicopathological parameters and patient survival. Cytotoxicity of mTOR inhibitor everolimus and EGFR inhibitor erlotinib alone and in combination was assessed using growth inhibition assay in NCI-H720 AC and SHP-77 LCNEC cells. Cell cycle phase distribution was determined by FACS. Apoptosis-associated activation of caspase-3/7 was measured by Caspase-Glo® assay. Activity status of EGFR and mTOR pathway components was analyzed by immunoblotting. Results: Activation of the EGFR/AKT/mTOR axis could be demonstrated in all entities and was significantly increased in higher grade tumors. Neoadjuvant chemotherapy correlated significantly with p-AKT expression and p-ERK loss. Erlotinib combined with everolimus exerted synergistic combination effects in AC and LCNEC cells by induction of apoptosis, while cell cycle phase distribution remained unaffected. These effects could be explained by synergistic downregulation of phospho-mTOR, phospho-p70S6 kinase and phospho-AKT expression by everolimus and erlotinib. Conclusions: Our study indicates that EGFR and mTOR are clinically important targets in bronchial neuroendocrine tumors, and further in vivo and clinical exploration of combined inhibition is warranted.[7] |
| Molecular Formula |
C22H23N3O4.HCL
|
|---|---|
| Molecular Weight |
429.90
|
| Exact Mass |
429.145
|
| Elemental Analysis |
C, 61.47; H, 5.63; Cl, 8.25; N, 9.77; O, 14.89
|
| CAS # |
183319-69-9
|
| Related CAS # |
Erlotinib-d6 hydrochloride;1189953-78-3;Erlotinib;183321-74-6;Erlotinib mesylate;248594-19-6;Erlotinib-13C6 hydrochloride;1210610-07-3;Erlotinib-d6;1034651-23-4
|
| PubChem CID |
176871
|
| Appearance |
White to off-white solid powder
|
| Boiling Point |
553.6ºC at 760 mmHg
|
| Melting Point |
223-225ºC
|
| Flash Point |
288.6ºC
|
| Vapour Pressure |
4.52E-12mmHg at 25°C
|
| LogP |
4.28
|
| Hydrogen Bond Donor Count |
2
|
| Hydrogen Bond Acceptor Count |
7
|
| Rotatable Bond Count |
11
|
| Heavy Atom Count |
30
|
| Complexity |
525
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
Cl[H].O(C([H])([H])C([H])([H])OC([H])([H])[H])C1C([H])=C2C(=NC([H])=NC2=C([H])C=1OC([H])([H])C([H])([H])OC([H])([H])[H])N([H])C1=C([H])C([H])=C([H])C(C#C[H])=C1[H]
|
| InChi Key |
GTTBEUCJPZQMDZ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C22H23N3O4.ClH/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22;/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25);1H
|
| Chemical Name |
N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine;hydrochloride
|
| Synonyms |
NSC718781 HCl; NSC-718781 HCl; CP358774 HCl, NSC 718781 HCl; erlotinib HCl; Tarceva; N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine hydrochloride; OSI-774; OSI 774; Erlotinib (Hydrochloride); CP-358774 HCl; CP 358774 HCl; OSI-774 HCl; OSI 774 HCl; OSI774 HCl; Erlotinib hydrochloride
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 0.5 mg/mL (1.16 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 5.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 0.5 mg/mL (1.16 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 5.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: 0.5 mg/mL (1.16 mM) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Solubility in Formulation 4: 15% Captisol: 15 mg/mL |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3261 mL | 11.6306 mL | 23.2612 mL | |
| 5 mM | 0.4652 mL | 2.3261 mL | 4.6522 mL | |
| 10 mM | 0.2326 mL | 1.1631 mL | 2.3261 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
GDC-0449 and Erlotinib Hydrochloride With or Without Gemcitabine Hydrochloride in Treating Patients With Metastatic Pancreatic Cancer or Solid Tumors That Cannot Be Removed by Surgery
CTID: NCT00878163
Phase: Phase 1 Status: Active, not recruiting
Date: 2024-09-19
|
 |
 |