
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg | |||
| 500mg | |||
| Other Sizes |
Purity: ≥98%
Elacridar HCl (formerly also known as GF120918; GF-120918; GW0918; GG918; GW120918) is a novel and potent P-glycoprotein (P-gp or MDR-1) and BCRP inhibitor. Elacridar has been used both in vitro and in vivo as a tool inhibitor of P-gp to investigate the role of transporters in the disposition of various test molecules. In vitro, GF120918A demonstrated high plasma protein binding across species, although a definitive protein binding evaluation was precluded by poor recovery, particularly in buffer and in mouse, rat, and dog plasma.
| Targets |
P-glycoprotein (Pgp); breast cancer resistance protein (BCRP)
|
|---|---|
| ln Vitro |
The cell viability of 786-O cells is inhibited by elacridar hydrochloride (0.001-1 μM; 2 h)[2]. P-glycoprotein and ABCG2 protein expression levels in MCF-7 and 786-O cell lines are impacted by elacridar hydrochloride (5 μM; 24 h)[2]. In MCF-7 and 786-O cells, elacridar hydrochloride (5 μM; 24 h) influences the intracellular accumulation of 99mTc-MIBI[2].
Elacridar (0.001-1 μM; 2 h) reduces the viability of 786-O cells [2]. Elacridar (5 μM; 24 h) modulates P-glycoprotein and ABCG2 protein expression levels in MCF-7 and 786-O cell lines [2]. Elacridar (5 μM; 24 h) impacts 99mTc-MIBI intracellular accumulation in MCF-7 and 786-O cells [2]. Researchers hypothesized that inhibition of multidrug resistant transporters by Elacridar (dual inhibitor of P-glycoprotein and ABCG 2) might overcome sunitinib resistance in experimental renal cell carcinoma. Human renal carcinoma cell lines 786-O, ACHN, and Caki-1 were treated with sunitinib or elacridar alone, or in combination. It was showed that Elacridar significantly enhanced sunitinib cytotoxicity in 786-O cells. P-glycoprotein activity, confirmed by P-glycoprotein function assay, was found to be inhibited by elacridar. ABCG2 expression was low in all renal carcinoma cell lines, and was suppressed only by combination treatment in 786-O cells. ABCG2 function was inhibited by sunitinib alone or combination with elacridar but not elacridar alone. These findings suggest that sunitinib resistance involves multidrug resistance transporters, and in combination with elacridar, can be reversed in renal carcinoma cells by P-glycoprotein inhibition. Elacridar sensitizes 786-O cells to sunitinib-induced cytotoxicity [2] In this assay, we evaluated the efficacy of elacridar in reversing multidrug resistance. Combined treatment of Elacridar and sunitinib decreased cell viability of 786-O and ACHN significantly compared to each single treatment, and it was elacridar concentration dependent manner (Fig. 1). These effects were not observed in MCF-7 or Caki-1 cells under the same treatments, including those given in combination. These results indicate that elacridar is capable of reversing P-glycoprotein and/or ABCG2-mediated multidrug resistance associated with the clinical anticancer agent sunitinib. Detection of P-glycoprotein and ABCG2 by western blotting [2] Western blot analysis showed a differential expression pattern of P-glycoprotein and ABCG2 proteins among cells. As shown in Fig. 3, the result was consistent with our speculation that the expression of P-glycoprotein was higher in 786-O cells than in other cell lines, although it was not significantly different. In addition, P-glycoprotein expression increased 2-fold in 786-O cells under hypoxia. In Caki-1 and MCF-7 cells, its expression remained stable. ABCG2 protein was relatively low in both RCC cells (Caki-1 and 786-O) compared to MCF-7, it was significantly different under hypoxic condition. Moreover, the effect of sunitinib and elacridar on the expression of P-glycoprotein (Fig. 4A) and ABCG2 (Fig. 4B) was investigated. Both drugs were tested at a concentration of 5 µM as a single treatment, or in combination (each 5 µM). As shown in Fig. 5, the combination of sunitinib and elacridar led to a significant reduction in ABCG2 expression in 786-O, suggesting that elacridar was able to inhibit the overexpression of this protein stimulated by sunitinib, while P-glycoprotein expression was not altered. In vitro imaging of multidrug resistance P-glycoprotein transport function using 99m Tc-MIBI [2] To investigate the potential mechanisms by which Elacridar sensitizes 786-O cells to sunitinib, we used a substrate of P-glycoprotein, 99mTc-MIBI as a radiotracer to visualize the expression of functional P-glycoprotein. MCF-7 and 786-O cells were treated with predetermined concentrations of sunitinib (5 µM) or elacridar (5 µM) alone, or in combination (each 5 µM), for 24 h. Cells were treated with 99mTc-MIBI, and gamma radiation was counted to determine the accumulation of 99mTc-MIBI in each cell line. As a result, intact MCF-7 cells showed higher accumulation of 99mTc-MIBI, while 786-O cells showed almost negligible amounts of the radiotracer (Fig. 5A). These results suggest that 786-O cells are better at extracting P-glycoprotein, which is consistent with the results from western blot analysis which showed that 786-O cells had higher expression of this transporter compared to MCF-7 cells (Fig. 3). As predicted, we noticed a dose-dependent increase in the intracellular accumulation of 99mTc-MIBI in 786-O cells following exposure to Elacridar as a single treatment or in combination with sunitinib (Fig. 5B). Single treatment of sunitinib resulted in a slight increase in the level of intracellular 99mTc-MIBI in 786-O cells, although this effect was less pronounced than that following elacridar treatment. The increase in 99mTc-MIBI accumulation over 60 min was time dependent. We observed an approximate 7-fold difference in the accumulation of 99mTc-MIBI between naïve 786-O cells and in combination-treated cells at 60 min. As predicted, the treatment of MCF-7 cells with elacridar had no effect on the intracellular 99mTc-MIBI accumulation. Neither sunitinib nor elacridar altered the intracellular levels of 99mTc-MIBI in Caki-1 cells, where P-glycoprotein expression was relatively low compared with that in 786-O cells (data not shown). Flow cytometry-based approach for detection of expression and function of ABCG2 transporter [2] In order to assess the functionality of the ABCG2 transporter in 786-O cells, and to evaluate the potency of sunitinib and Elacridar as an in vitro inhibitor of this transporter, we used Ph A, a chlorophyll catabolite, which is reported to be a specific probe to measure ABCG2 function (Robey et al., 2004). Flow cytometry analysis revealed that treating cells with 25 µM elacridar increased the accumulation of Ph A in 786-O cells around 5-fold. Moreover, at a dose of 5 µM, sunitinib led to the Ph A accumulation of 10 times more than in untreated controls (Fig. 6). The combination treatment seemed to further increase the accumulation compared with elacridar single treated cells, although this was almost the same level as that observed in sunitinib single treated cells. The assay was also performed in MCF-7, and exposure to sunitinib treatment alone increased the accumulation of Ph A 12-fold. ACHN cells treated with sunitinib accumulated up to 14.5 times more Ph A than the controls (data not shown). These results suggest that sunitinib causes a dose-dependent increase in the intracellular Ph A levels in RCC cells. In this case, elacridar was less active than sunitinib, although it caused a more potent inhibition of P-glycoprotein function. |
| ln Vivo |
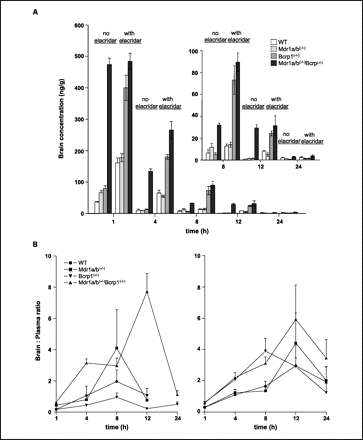
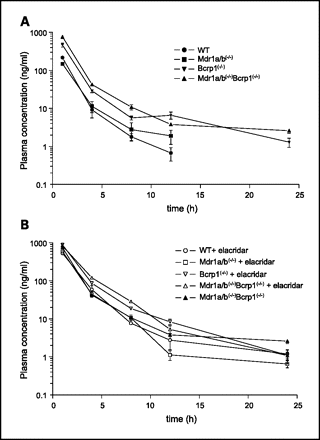
Elacridar hydrochloride (100 mg/kg; intraperitoneally) distributes differently in plasma and the brain[1]. 1.19 Elacridar hydrochloride plasma pharmacokinetic parameters in mice[1]. AUC0-inf (μg·min/ml) 1460 90.3 161.4 F 0.22 0.013 1 Mice PO 100 mg/kg Mice IP 100 mg/kg Mice IV 2.5 mg/kg CL/F (ml/min) 2.05 33.2 0.46 Vd/F (liter) 3.5 12.3 0.17 t1/2 (h) 20 4.3 4.4
Intravenous Administration of Elacridar. [1] The disposition of elacridar in plasma and brain was studied in FVB wild-type mice after an intravenous injection. The plasma concentrations showed a bi-exponential decline indicating distinct distribution and elimination phases (Fig. 1A). Concentrations rapidly reached peak levels in brain within 0.5 h after intravenous dosing with the maximal concentration (Cmax) observed at 0.5 h, the first measured time point. The brain-to-plasma concentration ratio was high at the initial time points (up to 2 h) and decreased thereafter as concentrations in brain declined more rapidly compared with those in plasma (Fig. 1B). This is consistent with the observed terminal t1/2 of 4.4 h in plasma and 1.5 h in brain (Table 1). The total plasma clearance was estimated to be 0.46 ml/min. The blood flow to the liver for a 20-g mouse is 1.8 ml/min (Davies and Morris, 1993), meaning elacridar has at most low to moderate hepatic extraction in the mouse. The AUC from time zero to infinity (AUC0-inf) was 161 μg · min/ml in plasma and 131 μg · min/ml in brain. The resulting brain Kp ratio was 0.82, indicating that after intravenous dosing at 2.5 mg/kg, there is approximately equal partitioning of elacridar into the brain as plasma. Intraperitoneal Administration of Elacridar. [1] The plasma and brain concentrations after an intraperitoneal dose of 100 mg/kg were measured in FVB wild-type mice. After intraperitoneal dosing, brain concentrations were significantly lower than plasma concentrations at all measured time points, except at 4 h after the dose (Fig. 2A). The corresponding brain-to-plasma concentration ratios remained less than one at all measured time points (Fig. 2B). It is important to note that the plasma concentrations after intravenous administration at which we observe a greater than one brain-to-plasma concentration ratio are higher than the maximal plasma concentrations seen after intraperitoneal dosing. The brain distribution ratio (i.e., the partition coefficient) of elacridar is likely to be dependent on its plasma levels. The observed Cmax in plasma after the intraperitoneal dose was 0.295 ± 0.06 μg/ml, and that in brain was 0.061 ± 0.024 μg/ml. The apparent plasma clearance (Cl/F) was estimated to be 33 ml/min by noncompartmental analysis. The AUC0-inf in plasma was 90.3 and 43.5 μg · min/ml in the brain. The elimination-phase t1/2 of elacridar after noncompartmental analysis was estimated to be 4.3 in plasma and 9.2 h in brain. The Kp ratio was 0.48, indicating that the partitioning into the brain was lower after intraperitoneal administration, even at a dose 40 times higher, compared with intravenous administration. Oral Administration of Elacridar. [1] The brain and plasma pharmacokinetics of elacridar were studied in FVB wild-type mice after a 100-mg/kg dose administered orally. The brain concentrations were lower than plasma concentrations until 1 h after the dose, after which the brain concentrations were several-fold higher than plasma concentrations (Fig. 3A). The brain-to-plasma ratio after oral administration was less than one for initial time points and then increased, reaching a maximum of approximately 6 at 4 h after the dose, before showing a slow decline (Fig. 3B). Noncompartmental analysis of plasma and brain concentration-time data showed that the plasma AUC0-inf was 1460 μg · min/ml and the brain AUC0-inf was 6296 μg · min/ml (Table 1). The Cmax in brain after oral dosing was 4.34 ± 0.79 μg/ml, significantly higher than that seen after intraperitoneal dosing. The time to reach Cmax in plasma was 4 h, indicating slow dissolution and absorption from the gut. After oral dosing, the brain Kp was found to be 4.31, suggesting that a high dose of elacridar administered orally gives high distribution in the brain. The t1/2 of the drug in the brain after the oral dose mirrored its plasma t1/2, with the values being 19.8 and 15.6 h, respectively. Determination of Bioavailability after Intraperitoneal and Oral Administration. [1] Absolute bioavailability was determined as a ratio of dose-normalized AUC after intraperitoneal or oral administration to dose-normalized AUC after intravenous administration (Table 2). The bioavailability after intraperitoneal administration was 1.3%, and that after oral administration was 22% with the suspension. The bioavailability after intraperitoneal dosing was very poor, indicating that intraperitoneal may not be a favorable route of administration to get reproducible plasma and brain exposure of elacridar using this simple suspension formulation. The bioavailability of elacridar after oral administration was higher than that after an equivalent dose administered intraperitoneally; this could be due to enhanced dissolution in the gut through greater solvent availability or micellar effects of bile salts. The t1/2 of elacridar after oral administration was approximately 5 times longer than that after intravenous or intraperitoneal administration. There is a possibility that there is nonlinearity in the absorption, distribution, and possibly elimination of elacridar. However, the bioavailability has been calculated with the assumption that there is no change in the clearance of the drug with increases in plasma exposure of the drug. |
| Enzyme Assay |
Analysis of Elacridar by Liquid Chromatography-Tandem Mass Spectrometry.
The concentrations of Elacridar in mouse plasma and brain were determined by high-performance liquid chromatography coupled with mass spectrometry. Frozen brain samples were thawed and homogenized with 3 volumes of 5% bovine serum albumin using a tissue homogenizer. Fifty microliters of plasma and 100 μl of brain homogenate were spiked with 20 ng of internal standard tyrphostin (AG 1478) and 100 μl of a buffer, pH 11 (0.1 M sodium hydroxide and 0.04 M sodium bicarbonate). Samples were extracted by vigorously vortexing with 1 ml of ethyl acetate for 5 min and then centrifugation at 7500 rpm for 15 min at 4°C. Six-hundred microliters of organic layer was transferred to microcentrifuge tubes and dried by a gentle stream of nitrogen. Samples were reconstituted in 100 μl of mobile phase and were transferred to autosampler vials. A 5-μl volume was injected using a temperature-controlled autosampler maintained at 10°C. Chromatographic analysis was performed using an Agilent Technologies Eclipse XDB-C18 RRHT threaded column (4.6 mm i.d. × 12.5 mm, 5 μ). The mobile phase was composed of acetonitrile: 20 mM ammonium formate (with 0.1% formic acid) (42:58 v/v) with a flow rate of 0.25 ml/min. The eluent was monitored using a Thermo Finnigan TSQ Quantum 1.5 detector. The instrument was equipped with an electrospray interface. The samples were ionized by the electrospray probe and analyzed in the positive ionization mode operating at a spray voltage of 4500 V for both and the internal standard. The spectrometer was programmed to allow the [MH+] ion of elacridar at m/z 564.6 and that of the internal standard at m/z 316.67 to pass through the first quadrupole (Q1) and into the collision cells (Q2). The collision energy was set at 39 V for elacridar and 9 V for tyrphostin. The product ions for elacridar (m/z 252.9) and the internal standard (m/z 300.9) were monitored through quadrupole 3 (Q3). The scan width and scan time for monitoring the two product ions were m/z 1.5 and 0.5 s, respectively. The assay was precise and linear over a range of 2.5 to 1500 ng/ml (CV was less than 10% over all concentrations).
Technetium-99m-labeled methoxyisobutyl isonitrile (99mTc-MIBI) in vitro uptake studies [2] 99mTc-MIBI was used as a transport substrate for P-glycoprotein (Piwnica-Worms et al., 1993). Na99mTcO4 was obtained in physiological saline as commercial 99Mo/99mTc generator eluate, and 99mTc-MIBI was prepared by adding Na99mTcO4 into a MIBI-kit vial and heating the mixture at 95 °C for 15 min. The radiopharmaceutical was diluted to 10 MBq/ml with saline. Cells were seeded into 100-mm culture dishes at a density of 2.0×106 per dish. After 24 h incubation, the medium was aspirated and replaced with 10 ml of culture medium with 5% FBS containing the appropriate concentrations of drug. After further 24 h incubation, supernatants were thoroughly aspirated, and single cell suspensions of 5.0×105 cells/ml were prepared. The time course of 99mTc-MIBI accumulation into MCF-7, Caki-1, ACHN, and 786-O cells was studied using single cell suspensions at 5.0×105 cells/ml incubated at 37 °C. Aliquots of 80 µl 99mTc-MIBI were added to vials containing 5 ml aliquots of cell suspension, and the cells were incubated at 37 °C for 1, 15, 30, 60, and 90 min after addition of the tracer. Duplicate samples of 400 µl were removed from each vial and transferred to 1.5 ml microcentrifuge tubes containing 500 µL ice cold saline and then centrifuged at 14,000g for 2 min. The supernatant was aspirated, and the cell pellet was carefully resuspended in 900 µl ice-cold saline and assayed for radioactivity using a gamma cell counter. Dose response curves were generated for sunitinib and Elacridar, and then the radioactivity was measured using a calibrated gamma counter (Ballinger et al., 1996). From the measurement of radioactivity in the cell pellet, the accumulation of radioactivity inside the cell, relative to that outside, (Cin/Cout) was determined. Flow cytometry to measure Ph A uptake [2] Expression of the ABCG2 transporter was determined by flow cytometry using the fluorescent compound pheophorbine A (Ph A), which was identified as an ABCG2 substrate based on Amcg2−/− knockout studies in mice. Cells were trypsinized and 3.0×105 cells were incubated in complete medium (phenol red-free DMEM with 10% FBS) with 10 µM Ph A with the desired concentration of sunitinib or Elacridar for 30 min at 37 °C in 5% CO2. The cells were then washed with cold complete medium, followed by incubation for 1 h at 37 °C in Ph A free complete medium. The cells were subsequently washed with cold complete medium and then incubated for 1 h at 37 °C in Ph A free complete medium. Finally, cells were washed twice with cold PBS and fluorescence was determined by flow cytometry. A total of 2.0×104 events were collected, and debris were eliminated by gating the forward versus side scatter. Intracellular fluorescence was analyzed with an excitation wavelength of 480 nm and an emission wavelength of 530 nm. |
| Cell Assay |
Cell Viability Assay[2]
Cell Types: 786-O cells Tested Concentrations: 2.5 and 5 μM Incubation Duration: 2 hrs (hours) Experimental Results: Dose-dependently inhibited cell viability of 786-O cells and demonstrated better inhibitory effect with sunitnib adding. Western Blot Analysis[2] Cell Types: MCF-7, Caki-1, and 786-O cell lines Tested Concentrations: 5 μM Incubation Duration: 24 hrs (hours) Experimental Results: Dreased P-glycoprotein protein expression level in 786-O cells and increased ABCG2 protein expression level in Caki-1 cells. Cell Viability Assay[2] Cell Types: MCF-7 and 786-O cell lines Tested Concentrations: 5 μM Incubation Duration: 24 hrs (hours) Experimental Results: Dose-dependently increased 99mTc-MIBI intracellular accumulation in MCF-7 and 786-O cells. Cytotoxicity assay (MTT assay) [2] To examine the combination effect of sunitinib and Elacridar against cell proliferation, 3.0×103 cells per well were seeded in a 96-well plate. After 24 h incubation, an optimum concentration gradient of sunitinib, Elacridar, or each combination was added to each well, followed by culturing for 48 h. Finally, cell viability was assessed using the proliferation reagent, MTT, according to the manufacturer׳s instructions. Control cells were treated with the vehicle only, 0.1% DMSO. After this final incubation, the medium was aspirated and precipitated formazan crystals were dissolved in DMSO (100 μL/well). The absorbance of each well was measured at 540 nm, and a reference wavelength of 650 nm was read with a multiskan JX microplate reader. Cell viability was calculated as percentage of the control value. Western blotting analysis [2] Expressions of multi-drug resistant proteins were examined by immuno-blotting. Cells were plated at a density of 2.5×105 cells per 60 mm dish and allowed to adhere for 24 h. The cells were then incubated with either sunitinib or Elacridar alone or in combination, in 5% FBS medium for further 24 h. Basal expressions in each cells were analyzed under normoxic or hypoxic conditions, while drug treated cells were analyzed under normoxic condition. Cells were lysed in ice cold buffer containing 0.5 M Tris–HCl, 100 mM β-glycerophosphate, 3 M NaCl, 50 mM EDTA, 100 mM Na3VO4, and 10% Triton-X, the lysates were cleared by centrifugation at 4 °C for 20 min at 12,000g, and protein concentrations were determined using the DC protein assay kit with BSA used as a standard. Protein lysates were loaded at concentrations of 25–30 µg for the ABC Sub-family Member 2, or 5060 µg for P-glycoprotein per lane and were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) before being electrophoretically transferred onto polyvinylidene fluoride (PVDF) membranes by tank transfer. Cell lysates analyzed for P-glycoprotein were resolved by 7.5% SDS-PAGE, while cell lysates analyzed for ABCG2 were resolved by 10% SDS-PAGE. Non-specific antibody binding was blocked by incubating the membranes in 5% skimmed milk. Membranes were then sequentially probed with primary monoclonal antibody (P-glycoprotein, mouse antibody: 1 h at room temperature at a 1:500 dilution in 1% milk, ABCG2, rabbit antibody) overnight at 4 °C at 1:1000 dilution in 1% milk. Subsequently, the membranes were washed with tris-buffered saline with Tween 20 (TBS-T) and were then incubated with horse-radish peroxidase (HRP)-conjugated secondary antibody (mouse secondary antibody: 1 h at room temperature at 1:1000 dilution in 1% milk, rabbit secondary antibody: 1 h at room temperature at 1:2000 dilution in 1% milk). |
| Animal Protocol |
Animal/Disease Models: FVB wild-type mice[1]
Doses: 100 mg/kg Route of Administration: intraperitoneal (ip)injection; 100 mg/kg once Experimental Results: Showd a higher concertation in brain than plasma except at 4 h after administration. Intravenous Administration of Elacridar. [1] The elacridar intravenous dosing solution was prepared on the day of the experiment by dissolving elacridar in a vehicle containing dimethyl sulfoxide, propylene glycol, and saline, 2:2:1 (v/v/v), at a concentration of 1.25 mg/ml. FVB wild-type mice were given intravenous doses of 2.5 mg/kg (2 μl volume/g b.wt.) in the tail vein. Blood and brain were collected at 0.5, 1, 2, 4, and 8 h after the dose (n = 4 at each time point). Animals were euthanized by use of a carbon dioxide chamber. Blood was collected by cardiac puncture, and plasma was obtained by centrifugation at 7500 rpm for 10 min at 4°C. The whole brain was quickly removed from skull and rinsed with ice-cold saline. Brains were immediately flash-frozen with liquid nitrogen. The specimens were stored at −80°C until analysis by liquid chromatography-tandem mass spectrometry. Intraperitoneal and Oral Administration of Elacridar. [1] Elacridar for intraperitoneal and oral dosing was prepared on the day of the experiment by preparing a stable suspension of elacridar, using 0.5% hydroxypropylmethylcellulose and 1% Tween 80 to obtain a 10-mg/ml formulation. Mice received an intraperitoneal dose of 100 mg/kg by injection into the peritoneal cavity. For oral administration, mice received a dose of 100 mg/kg by oral gavage. Blood and brain were sampled at 15 min, 0.5, 1, 2, 4, and 8 h after intraperitoneal dosing and at 0.5, 1, 2, 4, 8, 17, and 24 h after oral dosing. Plasma and brain samples were collected and processed in the manner described in the previous section. |
| ADME/Pharmacokinetics |
The objective of this study was to determine the bioavailability and disposition of elacridar (GF120918; N-(4-(2-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-isoquinolinyl)ethyl)phenyl)-9,10-dihydro-5-methoxy-9-oxo-4-acridine carboxamide) in plasma and brain after various routes of administration in the mouse. Elacridar is a potent inhibitor of P-glycoprotein and breast cancer resistance protein and has been used to examine the influence of these efflux transporters on drug distribution to brain. Friend leukemia virus strain B mice were administered 100 mg/kg elacridar either orally or intraperitoneally. The absolute bioavailability of elacridar after oral or intraperitoneal dosing was determined with respect to an intravenous dose of 2.5 mg/kg. At these doses, the absolute bioavailability was 0.22 for oral administration and 0.01 for intraperitoneal administration. The terminal half-life of elacridar was approximately 4 h after intraperitoneal and intravenous administration and nearly 20 h after oral dosing. The brain-to-plasma partition coefficient (Kp,brain) of elacridar increased as plasma exposure increased, suggesting saturation of the efflux transporters at the blood-brain barrier. The Kp,brain after intravenous, intraperitoneal, and oral dosing was 0.82, 0.43, and 4.31, respectively. The low aqueous solubility and high lipophilicity of elacridar result in poor oral absorption, most likely dissolution-rate-limited. These results illustrate the importance of the route of administration and the resultant plasma exposure in achieving effective plasma and brain concentrations of elacridar and can be used as a guide for future studies involving elacridar administration and in developing formulation strategies to overcome the poor absorption.[1]
Intravenous Administration of Elacridar. [1] The disposition of elacridar in plasma and brain was studied in FVB wild-type mice after an intravenous injection. The plasma concentrations showed a bi-exponential decline indicating distinct distribution and elimination phases (Fig. 1A). Concentrations rapidly reached peak levels in brain within 0.5 h after intravenous dosing with the maximal concentration (Cmax) observed at 0.5 h, the first measured time point. The brain-to-plasma concentration ratio was high at the initial time points (up to 2 h) and decreased thereafter as concentrations in brain declined more rapidly compared with those in plasma (Fig. 1B). This is consistent with the observed terminal t1/2 of 4.4 h in plasma and 1.5 h in brain (Table 1). The total plasma clearance was estimated to be 0.46 ml/min. The blood flow to the liver for a 20-g mouse is 1.8 ml/min (Davies and Morris, 1993), meaning elacridar has at most low to moderate hepatic extraction in the mouse. The AUC from time zero to infinity (AUC0-inf) was 161 μg · min/ml in plasma and 131 μg · min/ml in brain. The resulting brain Kp ratio was 0.82, indicating that after intravenous dosing at 2.5 mg/kg, there is approximately equal partitioning of elacridar into the brain as plasma. Intraperitoneal Administration of Elacridar. [1] The plasma and brain concentrations after an intraperitoneal dose of 100 mg/kg were measured in FVB wild-type mice. After intraperitoneal dosing, brain concentrations were significantly lower than plasma concentrations at all measured time points, except at 4 h after the dose (Fig. 2A). The corresponding brain-to-plasma concentration ratios remained less than one at all measured time points (Fig. 2B). It is important to note that the plasma concentrations after intravenous administration at which we observe a greater than one brain-to-plasma concentration ratio are higher than the maximal plasma concentrations seen after intraperitoneal dosing. The brain distribution ratio (i.e., the partition coefficient) of elacridar is likely to be dependent on its plasma levels. The observed Cmax in plasma after the intraperitoneal dose was 0.295 ± 0.06 μg/ml, and that in brain was 0.061 ± 0.024 μg/ml. The apparent plasma clearance (Cl/F) was estimated to be 33 ml/min by noncompartmental analysis. The AUC0-inf in plasma was 90.3 and 43.5 μg · min/ml in the brain. The elimination-phase t1/2 of elacridar after noncompartmental analysis was estimated to be 4.3 in plasma and 9.2 h in brain. The Kp ratio was 0.48, indicating that the partitioning into the brain was lower after intraperitoneal administration, even at a dose 40 times higher, compared with intravenous administration. Oral Administration of Elacridar. [1] The brain and plasma pharmacokinetics of elacridar were studied in FVB wild-type mice after a 100-mg/kg dose administered orally. The brain concentrations were lower than plasma concentrations until 1 h after the dose, after which the brain concentrations were several-fold higher than plasma concentrations (Fig. 3A). The brain-to-plasma ratio after oral administration was less than one for initial time points and then increased, reaching a maximum of approximately 6 at 4 h after the dose, before showing a slow decline (Fig. 3B). Noncompartmental analysis of plasma and brain concentration-time data showed that the plasma AUC0-inf was 1460 μg · min/ml and the brain AUC0-inf was 6296 μg · min/ml (Table 1). The Cmax in brain after oral dosing was 4.34 ± 0.79 μg/ml, significantly higher than that seen after intraperitoneal dosing. The time to reach Cmax in plasma was 4 h, indicating slow dissolution and absorption from the gut. After oral dosing, the brain Kp was found to be 4.31, suggesting that a high dose of elacridar administered orally gives high distribution in the brain. The t1/2 of the drug in the brain after the oral dose mirrored its plasma t1/2, with the values being 19.8 and 15.6 h, respectively. Determination of Bioavailability after Intraperitoneal and Oral Administration. [1] Absolute bioavailability was determined as a ratio of dose-normalized AUC after intraperitoneal or oral administration to dose-normalized AUC after intravenous administration (Table 2). The bioavailability after intraperitoneal administration was 1.3%, and that after oral administration was 22% with the suspension. The bioavailability after intraperitoneal dosing was very poor, indicating that intraperitoneal may not be a favorable route of administration to get reproducible plasma and brain exposure of elacridar using this simple suspension formulation. The bioavailability of elacridar after oral administration was higher than that after an equivalent dose administered intraperitoneally; this could be due to enhanced dissolution in the gut through greater solvent availability or micellar effects of bile salts. The t1/2 of elacridar after oral administration was approximately 5 times longer than that after intravenous or intraperitoneal administration. There is a possibility that there is nonlinearity in the absorption, distribution, and possibly elimination of elacridar. However, the bioavailability has been calculated with the assumption that there is no change in the clearance of the drug with increases in plasma exposure of the drug. |
| References |
|
| Additional Infomation |
Elacridar (GW120918) is an oral bioenhancer that targets multiple drug resistance in tumors. In many cases, the appearance of multidrug resistance in cancer is due to a change in expression of protein inhibitors. As of August 2007 elacridar was not listed on GSK's product pipeline. Development is assumed to have been discontinued.
Drug Indication For the treatment of solid tumors. Mechanism of Action P-glycoprotein is a well characterized human ABC-transporter of the MDR/TAP subfamily. It is an ATP-dependent efflux pump with broad substrate specificity. It likely evolved as a defence mechanism against harmful substances. Increased intestinal expression of P-glycoprotein can reduce the absorption of drugs that are substrates for P-glycoprotein. Thus, there is a reduced bioavailability, therapeutic plasma concentrations are not attained. Elacridar functions by inhibiting P-glycoprotein, resulting in an increased bioavailability of coadminstered drugs. Pharmacodynamics Elacridar is an oral bioenhancer that targets multiple drug resistance in tumors. In many cases, the appearance of multidrug resistance in cancer is due to a change in expression of protein inhibitors. Elacridar is a third-generation inhibitor of the transporters P-gp and BCRP (Hyafil et al., 1993; Witherspoon et al., 1996). It has been used to determine the influence of P-gp and BCRP on the brain distribution of drugs that are substrates for P-gp and BCRP (Breedveld et al., 2005; Bihorel et al., 2007; Chen et al., 2009; Agarwal et al., 2010, 2011b). It has also been suggested as a tool to improve the efficacy of drugs such as sunitinib in the treatment of glioma (Tang et al., 2012a). The objective of this study was to investigate the brain and plasma pharmacokinetics of elacridar in the mouse and determine its bioavailability after oral and intraperitoneal administration. The physicochemical properties of elacridar such as poor solubility and high lipophilicity could be responsible for the variability that is observed in plasma concentrations in this study. This interindividual variability has been observed previously in humans upon oral administration of elacridar (Kuppens et al., 2007) and was attributed to variability in dissolution of elacridar. In a study done in mice, the rate of dissolution of elacridar was also found to be a factor in limiting the plasma exposure of orally administered elacridar, despite increasing its dose (Ward and Azzarano, 2004). Oral dosing is a convenient route for drug administration in both rodents and humans, especially for chronic administration. It has been shown that brain penetration of the TKI sunitinib and its active metabolite is limited by P-gp and BCRP at the BBB. Oral administration of elacridar at a 100 mg/kg dose improved the brain penetration of sunitinib in wild-type mice by nearly 12-fold (Tang et al., 2012a,b). Both plasma and brain exposure of elacridar after a 100 mg/kg oral dose was significantly higher than that after intraperitoneal administration. The partition coefficient of elacridar in the brain after oral administration was 4.31, indicating good distribution of elacridar from systemic circulation into the brain after this oral dose. The elimination t1/2 after an oral dose of 100 mg/kg was close to 20 h, approximately 5-fold greater than the t1/2 after intraperitoneal or intravenous dosing. There could be several possible reasons for this phenomenon. The increased oral t1/2 could be due to a slow dissolution rate (given the poor aqueous solubility), which limits the rate of absorption and thus leads to an extended-release period. Thus, the observed terminal t1/2 might be a measure of a possible long absorption t1/2 (flip-flop kinetics). The finding that the observed Tmax in plasma was 8 h postdose supports this hypothesis. Another reason for the extended t1/2 could be a change in hepatic or renal clearance of the drug due to the higher plasma exposures of elacridar. However, elacridar is not a potent inhibitor of any cytochrome P450 enzymes in vitro (Ward and Azzarano, 2004), with the IC50 values in the micromolar range. The AUCplasma (dose-normalized) was greater after oral dosing versus intraperitoneal dosing, which resulted in a greater bioavailability. This may be explained by inhibition of intestinal P-gp by elacridar after its oral administration, which would also improve its absorption from the gastrointestinal tract, contributing to a higher bioavailability compared with intraperitoneal administration. Another possible explanation of this phenomenon could be enterohepatic recycling of elacridar upon oral administration, which could lead to a larger than expected AUC. Irrespective of these possibilities, the results show that high-dose administration of elacridar by the oral route can yield high exposures in both plasma and brain. Tang et al. (2012a) suggested that a 100-mg/kg dose could represent an effective dose in mice and indicated that there is no significant toxicity associated with administration of this dose. Although toxicity was not evaluated in the current study, there were no overt elacridar-related adverse effects after this single dose in mice. However, if a chronic dosing regimen is to be considered, the concerns for toxicity cannot be dismissed, especially considering the high plasma concentrations and the extended t1/2 of elacridar (which could lead to significant accumulation at steady state) that are seen with this dose. Intraperitoneal dosing is also an attractive option for chronic dosing, especially in preclinical studies. The mice that received elacridar intraperitoneally showed a much lower plasma exposure compared with oral administration after an equivalent dose. This may most likely be due to poor dissolution and/or absorption of the drug from the peritoneal cavity. The plasma and brain concentrations also showed significant interanimal variability. The intraperitoneal route of administration, though convenient to dose chronically in mice, resulted in reduced plasma exposure of elacridar; therefore, the applications of an intraperitoneal dosing regimen with the current suspension formulation are limited. In the current study, the Kp (AUCbrain/AUCplasma) ratio after an intraperitoneal dose of 100 mg/kg was 0.48. In a similar study by Padowski and Pollack (2010), an intraperitoneal dose of 10 mg/kg yielded a Kp of 0.0784. Taken together, these findings may indicate the presence of nonlinearity in plasma and brain exposures with increasing dose, especially after intraperitoneal dosing. Exposure after an intravenous dose was used as the reference for calculating the absolute bioavailability after the other two routes of administration. After an intravenous dose of 2.5 mg/kg, the AUC in of elacridar in plasma and brain were approximately equal, yielding a Kp ratio of 0.82 (Table 1). Despite observing this substantial brain distribution of elacridar after intravenous administration, intravenous dosing is not a viable option for chronic dosing in noncatheterized mice because of difficulties in performing repeated injections. Moreover, the dosing solution prepared for intravenous administration is unstable because it is prone to precipitation. The bioavailability of elacridar is limited by its poor physicochemical properties. The bioavailability of elacridar after oral administration of a high dose was approximately 22%, whereas that after intraperitoneal administration at the same dose was only approximately 1%. The higher AUCplasma after oral administration versus intraperitoneal administration could be due to the solubilizing effect of bile salts in the gut. Oral administration of elacridar, when using this simple suspension formulation, appears to be the most effective way to achieve plasma exposures necessary to effectively inhibit P-gp and BCRP at the BBB. There were significant findings in this study related to the brain penetration of elacridar after the different routes of administration. First, the Kp for elacridar (a measure of its brain distribution) was found to be dependent on its plasma exposure (see Fig. 4). When the plasma exposure was relatively high, as seen after oral and intravenous administration, the Kp ratio was greater than one, with the highest Kp ratio of ∼5 seen after oral dosing, which yielded the highest plasma exposure. The Kp ratio after the intraperitoneal route of administration, which resulted in a relatively low plasma exposure, was less than one. Second, the brain-to-plasma concentration ratios plotted as a function of time for all routes of administration showed an increase to a maximal value followed by a decrease (see Figs. 1B, 2B, and 3B). This was unexpected because after reaching a steady state in the tissue of distribution (pseudodistributional equilibrium), the brain-to-plasma concentration ratio should remain constant. One explanation for both these findings can be the active efflux of elacridar from the brain by P-gp and BCRP. Elacridar inhibits both of these transporters at the BBB, and it is possible that the mechanism behind the inhibitory action might be competitive (because of it being a substrate for the two transporters). This has been shown to be true in a study that used positron emission tomographic imaging and transporter knockout mice to study the influence of P-gp and BCRP on elacridar distribution at the BBB (Kawamura et al., 2011a,b). This finding is currently being further investigated in another study. However, it does explain why brain exposures are dependent on plasma concentrations. As the plasma concentrations drop below levels that are required to saturate efflux at the BBB, the net efflux from the brain will become greater than the passive diffusion into the brain. This would result in the brain concentrations decreasing more rapidly than the corresponding plasma concentration, ultimately resulting in a decreasing trend in brain-to-plasma ratios with respect to time.[1] Concurrent administration of elacridar with drugs that are substrates for P-gp and BCRP improves their distribution across the BBB and could lead to improved efficacy. The study of elacridar pharmacokinetics in the brain is important if we consider the issue of target cells that are present behind an intact BBB in the invasive rim of a brain tumor or the normal brain (Agarwal et al., 2011a). Distribution of elacridar in the brain tissue could possibly address the issue of target cells that express BCRP and P-gp and are therefore resistant to chemotherapy (Lu and Shervington, 2008). Elacridar in the brain could be effective in inhibiting P-gp and BCRP present on these cells, allowing the chemotherapeutic agents to act on them, possibly preventing recurrence of the tumor. A targeted approach for central nervous system delivery that employs elacridar could also reduce the dose required to achieve effective brain concentrations, thus reducing systemic toxicity. The use of elacridar has been limited in both preclinical and clinical situations partly because of its poor solubility and poor bioavailability. It is an unmet need to improve the bioavailability and solubility of elacridar. There are several possible methods that could be explored, including synthesis of water-soluble prodrugs, preparation of solid dispersions, and use of surfactants, cyclodextrins, and permeation enhancers. In summary, this study has examined the pharmacokinetics of elacridar in plasma and brain in mice and determined its bioavailability after different routes of administration. The results from these experiments can be used to help guide the selection of doses and routes of administration of elacridar for future studies.[1] |
| Molecular Formula |
C34H33N3O5.HCL
|
|
|---|---|---|
| Molecular Weight |
600.1
|
|
| Exact Mass |
599.219
|
|
| Elemental Analysis |
C, 68.05; H, 5.71; Cl, 5.91; N, 7.00; O, 13.33
|
|
| CAS # |
143851-98-3
|
|
| Related CAS # |
Elacridar;143664-11-3
|
|
| PubChem CID |
170320
|
|
| Appearance |
Light yellow to yellow solid powder
|
|
| Boiling Point |
701.6ºC at 760 mmHg
|
|
| Flash Point |
378.1ºC
|
|
| Vapour Pressure |
1.57E-19mmHg at 25°C
|
|
| LogP |
6.373
|
|
| Hydrogen Bond Donor Count |
3
|
|
| Hydrogen Bond Acceptor Count |
7
|
|
| Rotatable Bond Count |
8
|
|
| Heavy Atom Count |
43
|
|
| Complexity |
925
|
|
| Defined Atom Stereocenter Count |
0
|
|
| SMILES |
COC1=CC=CC2=C1NC3=C(C2=O)C=CC=C3C(=O)NC4=CC=C(C=C4)CCN5CCC6=CC(=C(C=C6C5)OC)OC.Cl
|
|
| InChi Key |
IQOJZZHRYSSFJM-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C34H33N3O5.ClH/c1-40-28-9-5-7-26-32(28)36-31-25(33(26)38)6-4-8-27(31)34(39)35-24-12-10-21(11-13-24)14-16-37-17-15-22-18-29(41-2)30(42-3)19-23(22)20-37;/h4-13,18-19H,14-17,20H2,1-3H3,(H,35,39)(H,36,38);1H
|
|
| Chemical Name |
N-[4-[2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)ethyl]phenyl]-5-methoxy-9-oxo-10H-acridine-4-carboxamide;hydrochloride
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: 2.08 mg/mL (3.47 mM) in 10% DMSO + 40% PEG300 +5% Tween-80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 + to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6664 mL | 8.3319 mL | 16.6639 mL | |
| 5 mM | 0.3333 mL | 1.6664 mL | 3.3328 mL | |
| 10 mM | 0.1666 mL | 0.8332 mL | 1.6664 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
 |
|---|
 |