
| Size | Price | Stock | Qty |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| 1g | |||
| Other Sizes |
Purity: ≥98%
Combretastatin A4 (CA-4; CRC-87-09; Combretastatin A-4) is a highly potent tubulin/microtubule inhibitor or microtubule polymerization destablizer with potential antitumor activity. It belongs to the so called microtubule-targeting agent (MTA) or microtubule disrupting agent and acts by binding to β-tubulin with a Kd of 0.4 μM. It has been in clinical trials for treating various cancers.
| Targets |
Microtubule; tubulin polymerization; β-tubulin (Kd = 0.4 μM)
|
||
|---|---|---|---|
| ln Vitro |
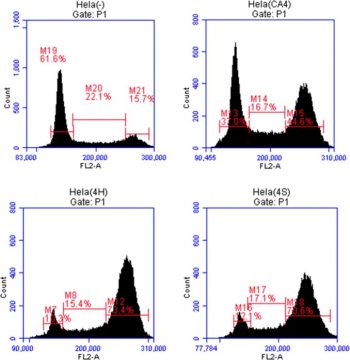
Forward scatter is greatly reduced and the proportion of Annexin-V bound cells is significantly increased when combretastatin A4 phosphate (≥ 50 μM) is used. The amount of hemolysis is not considerably increased by combretastatin A4 phosphate. Combretastatin A4 phosphate at concentrations of several hundred μM markedly increased Fluo3 fluorescence. When extracellular Ca2+ is removed, the effect of Combretastatin A4 phosphate (100 μM) on Annexin-V binding is greatly reduced but not completely eliminated. ROS and ceramide are not significantly increased by combretastatin A4 phosphate (≥ 50 μM), but it does dramatically lower GSH abundance and ATP levels [2]. Strong synergistic cytotoxicity was demonstrated by polymer capsules co-encapsulating doxorubicin-combretastatin-A4 phosphate (1:10) against human nasopharyngeal epithelial carcinoma (KB) cells [3]. The expression of these important molecules and the quantity of VM in 3-D cells are unaffected by pretreatment with combretastatin A4 phosphate [4].
A 48 hours exposure of human erythrocytes to Combretastatin A4/CA4P (≥ 50 µM) significantly increased the percentage of annexin-V-binding cells and significantly decreased forward scatter. Combretastatin A4/CA4P did not appreciably increase hemolysis. Hundred µM CA4P significantly increased Fluo3-fluorescence. The effect of CA4P (100 µM) on annexin-V-binding was significantly blunted, but not abolished, by removal of extracellular Ca2+. CA4P (≥ 50 µM) significantly decreased GSH abundance and ATP levels but did not significantly increase ROS or ceramide. Conclusions: Combretastatin A4CA4P triggers cell shrinkage and phospholipid scrambling of the erythrocyte cell membrane, an effect at least in part due to entry of extracellular Ca2+ and energy depletion.[2] In vitro model of three-dimensional cultures was used to test the effect of Combretastatin A4/CA4P on the tube formation of Walker 256 cells. Western blot analysis was conducted to assess the expression of hypoxia-inducible factor (HIF)-1α and VM-associated markers.Under hypoxic conditions for 48 h in vitro, W256 cells formed VM network associated with increased expression of VM markers. Pretreatment with CA4P did not influence the amount of VM in 3-D culture as well as the expression of these key molecules [4]. |
||
| ln Vivo |
Thirty minutes after the treatment, rats given 120 mg/10 mL/kg of Combretastatin A4 disodium phosphate had more DBP and MBP. Rats treated with Combretastatin A4 disodium phosphate 120 mg/10 mL/kg showed the following toxicokinetic characteristics for both Combretastatin A4 and its phosphate: Cmax, T1/2, and AUC0-inf values of Combretastatin A4 were 156± 13 μM, 5.87±1.69 h, and 89.4±10.1 h·μM[1]. W256 tumors showed a substantial intratumoral hypoxia following combretastatin A4 phosphate therapy, which was associated by an increase in VM development. Tumor growth was delayed with cercopetastatin A4 phosphate for a mere two days, however the growth of the tumor quickly resumed. Positive correlations were seen between the VM density and the tumor weight and volume on day 8. Through the HIF-1α/EphA2/PI3K/matrix metalloproteinase (MMP) signaling pathway, cercetastatin A4 phosphate stimulates hypoxia and VM formation in W256 tumors, which impairs tumor renewal [4].
In this study, we designed biodegradable polymersomes for co-delivery of an antiangiogenic drug Combretastatin A4 phosphate (CA4P) and doxorubicin (DOX) to collapse tumor neovasculature and inhibit cancer cell proliferation with the aim to achieve synergistic antitumor effects. The polymersomes co-encapsulating DOX and CA4P (Ps-DOX-CA4P) were prepared by solvent evaporation method using methoxy poly(ethylene glycol)-b-polylactide (mPEG-PLA) block copolymers as drug carriers. The resulting Ps-DOX-CA4P has vesicles shape with uniform sizes of about 50 nm and controlled co-encapsulation ratios of DOX to CA4P. More importantly, Ps-DOX-CA4P (1:10) showed strong synergistic cytotoxicity (combination index CI = 0.31) against human nasopharyngeal epidermal carcinoma (KB) cells. Furthermore, Ps-DOX-CA4P accumulated remarkably in KB tissues xenografts in nude mice. Consistent with these observations, Ps-DOX-CA4P (1:10) achieved significant antitumor potency because of fast tumor vasculature disruption and sustained tumor cells proliferation inhibition in vivo. The overall findings indicate that co-delivery of an antiangiogenic drug and a chemotherapeutic agent in polymersomes is a potentially promising strategy for cancer therapy. [3] In vivo, W256 tumors showed marked intratumoral hypoxia after Combretastatin A4/CA4P treatment, accompanied by increased VM formation. CA4P exhibited only a delay in tumor growth within 2 days but rapid tumor regrowth afterward. VM density was positively related to tumor volume and tumor weight at day 8. CA4P causes hypoxia which induces VM formation in W256 tumors through HIF-1α/EphA2/PI3K/matrix metalloproteinase (MMP) signaling pathway, resulting in the consequent regrowth of the damaged tumor [4]. |
||
| Enzyme Assay |
In Vitro Tubulin Polymerization Assay[5,6]
According to the method described by Wang et al.,porcine brain tubulins (>97% pure) were mixed with general tubulin buffer (80 mM PIPES, 2.0 mM MgCl2, 0.5 mM EGTA, and 1 mM GTP) to reach a final concentration of 3 mg/mL at 4 °C. The tubulin polymerization assay was incubated at 37 °C in a SYNERGY 4 Microplate Reader immediately after mixing tubulin protein solution and the test compounds in a 96-well plate and monitored every 30 s for 65 min at 340 nm. The experiment was performed in duplicates with paclitaxel as a positive control for tubulin polymerization, and colchicine and ABI-274 as positive controls for tubulin depolymerization. SPR for Affinity Assay[5,6] Binding affinity with tubulin was analyzed using SPR technology in a Reichert4SPR system equipped with a dextran SPR sensor chip (Reichert Polycarboxylate Hydrogel Chip P/N 13206067). Then, 50 μg/mL tubulin was immobilized to the sensor chip surface to attain 12 000 μRIU. One of the four flow cells on the chip was left free as a negative control. 4v or colchicine at different concentrations was injected over the sensor chip surface for association analysis, followed by dissociation analysis. The experiment data were obtained at 25 °C with a running buffer PBST (8 mM Na2HPO4, 136 mM NaCl, 2 mM KH2PO4, 2.6 mM KCl, and 0.05% (v/v) Tween 20, pH 7.4). The equilibrium dissociation constant (KD) was calculated by a steady-state fitting mode with TraceDrawer software. |
||
| Cell Assay |
Evaluation of cellular impedance [1]
Analysis of cellular impedance of hiPS-CMs using an xCELLigence Cardio Analyzer was performed with reference to and modification of the methods in earlier studies. Briefly, iCell hiPS-CMs were purchased from Cellular Dynamics International. hiPS-CMs were thawed and cultured in 96-well xCELLigence Cardio E-plates at 20,000 cells/well and 37°C in 5% CO2, using plating medium and maintenance medium specifically for iCell hiPS-CMs, according to the manufacturer’s protocol. During the incubation period, the impedance values were monitored continuously using an xCELLigence Cardio Analyzer according to the manufacturer’s instructions. Impedance was continuously sampled at 12.9 ms intervals and monitored at every measurement point with a 20 second sweep duration. After incubation for 14 days, test compounds (100 nM, 1 μM, and 10 μM CA4DP; 100 nM, 1 μM, and 10 μM Combretastatin A4/CA4; and 0.1% H2O for CA4DP or 0.1% DMSO for CA4 [vehicle]) (n = 3 well) were added to the culture. Then, impedance cell index (CI) and beating rate16, 18, 20 were calculated using dedicated software. Data for CI and beating rate were normalized by the value immediately before the addition of test compounds. The CI for 36 hours after administration was used to detect cytotoxic effects. The beating rate at 15 minutes, 3 hours, and 12 hours after administration were used to detect changes in contractility. Background/aims: Combretastatin A4 phosphate disodium (CA4P) is utilized for the treatment of malignancy. The substance has previously been shown to trigger suicidal cell death or apoptosis. Similar to apoptosis of nucleated cells, erythrocytes may enter suicidal death or eryptosis, characterized by cell shrinkage and cell membrane scrambling with phosphatidylserine translocation to the erythrocyte surface. Stimulators of eryptosis include increase of cytosolic Ca2+ activity ([Ca2+]i), ceramide, oxidative stress and ATP depletion. The present study explored, whether CA4P induces eryptosis and, if so, to gain insight into mechanisms involved. Methods: Flow cytometry has been employed to estimate phosphatidylserine exposure at the cell surface from annexin-V-binding, cell volume from forward scatter, [Ca2+]i from Fluo3-fluorescence, reactive oxygen species (ROS) abundance from DCF fluorescence, glutathione (GSH) abundance from CMF fluorescence and ceramide abundance from fluorescent antibodies. In addition cytosolic ATP levels were quantified utilizing a luciferin-luciferase-based assay and hemolysis was estimated from hemoglobin concentration in the supernatant [2]. |
||
| Animal Protocol |
|
||
| ADME/Pharmacokinetics |
Metabolism / Metabolites
Combretastatin A4 has known human metabolites that include (2S,3S,4S,5R)-3,4,5-trihydroxy-6-[2-methoxy-5-[(Z)-2-(3,4,5-trimethoxyphenyl)ethenyl]phenoxy]oxane-2-carboxylic acid. |
||
| References |
|
||
| Additional Infomation |
Combretastatin A4 is a stilbenoid.
Combretastatin A4 has been reported in Combretum caffrum with data available. Combretastatin A-4 is an inhibitor of microtubule polymerization derived from the South African willow bush which causes mitotic arrest and selectively targets and reduces or destroys existing blood vessels, causing decreased tumor blood supply. See also: Fosbretabulin (annotation moved to). Combretastatin A-4 is an inhibitor of microtubule polymerization derived from the South African willow bush which causes mitotic arrest and selectively targets and reduces or destroys existing blood vessels, causing decreased tumor blood supply. Histopathological and electrocardiographic features of myocardial lesions induced by combretastatin A4 disodium phosphate (CA4DP) were evaluated, and the relation between myocardial lesions and vascular changes and the direct toxic effect of CA4DP on cardiomyocytes were discussed. We induced myocardial lesions by administration of CA4DP to rats and evaluated myocardial damage by histopathologic examination and electrocardiography. We evaluated blood pressure (BP) of CA4DP-treated rats and effects of CA4DP on cellular impedance-based contractility of human induced pluripotent stem cell-derived cardiomyocytes (hiPS-CMs). The results revealed multifocal myocardial necrosis with a predilection for the interventricular septum and subendocardial regions of the apex of the left ventricular wall, injury of capillaries, morphological change of the ST junction, and QT interval prolongation. The histopathological profile of myocardial lesions suggested that CA4DP induced a lack of myocardial blood flow. CA4DP increased the diastolic BP and showed direct effects on hiPS-CMs. These results suggest that CA4DP induces dysfunction of small arteries and capillaries and has direct toxicity in cardiomyocytes. Therefore, it is thought that CA4DP induced capillary and myocardial injury due to collapse of the microcirculation in the myocardium. Moreover, the direct toxic effect of CA4DP on cardiomyocytes induced myocardial lesions in a coordinated manner.[1] The purpose of this study was to investigate the effect of combretastatin A4 phosphate (CA4P) on vasculogenic mimicry (VM) channel formation in vitro and in vivo after a single-dose treatment and the underlying mechanism involved in supporting VM. In vitro model of three-dimensional cultures was used to test the effect of CA4P on the tube formation of Walker 256 cells. Western blot analysis was conducted to assess the expression of hypoxia-inducible factor (HIF)-1α and VM-associated markers. W256 tumor-bearing rat model was established to demonstrate the effect of CA4P on VM formation and tumor hypoxia by double staining and a hypoxic marker pimonidazole. Anti-tumor efficacy of CA4P treatment was evaluated by tumor growth curve. Under hypoxic conditions for 48 h in vitro, W256 cells formed VM network associated with increased expression of VM markers. Pretreatment with CA4P did not influence the amount of VM in 3-D culture as well as the expression of these key molecules. In vivo, W256 tumors showed marked intratumoral hypoxia after CA4P treatment, accompanied by increased VM formation. CA4P exhibited only a delay in tumor growth within 2 days but rapid tumor regrowth afterward. VM density was positively related to tumor volume and tumor weight at day 8. CA4P causes hypoxia which induces VM formation in W256 tumors through HIF-1α/EphA2/PI3K/matrix metalloproteinase (MMP) signaling pathway, resulting in the consequent regrowth of the damaged tumor.[4] We recently reported the crystal structure of tubulin in complex with a colchicine binding site inhibitor (CBSI), ABI-231, having 2-aryl-4-benzoyl-imidazole (ABI). Based on this and additional crystal structures, here we report the structure-activity relationship study of a novel series of pyridine analogues of ABI-231, with compound 4v being the most potent one (average IC50 ∼ 1.8 nM) against a panel of cancer cell lines. We determined the crystal structures of another potent CBSI ABI-274 and 4v in complex with tubulin and confirmed their direct binding at the colchicine site. 4v inhibited tubulin polymerization, strongly suppressed A375 melanoma tumor growth, induced tumor necrosis, disrupted tumor angiogenesis, and led to tumor cell apoptosis in vivo. Collectively, these studies suggest that 4v represents a promising new generation of tubulin inhibitors. [5] Novel ABI-III compounds were designed and synthesized based on our previously reported ABI-I and ABI-II analogues. ABI-III compounds are highly potent against a panel of melanoma and prostate cancer cell lines, with the best compound having an average IC(50) value of 3.8 nM. They are not substrate of Pgp and thus may effectively overcome Pgp-mediated multidrug resistance. ABI-III analogues maintain their mechanisms of action by inhibition of tubulin polymerization.[6] |
| Molecular Formula |
C18H20O5
|
|
|---|---|---|
| Molecular Weight |
316.35
|
|
| Exact Mass |
316.131
|
|
| Elemental Analysis |
C, 68.34; H, 6.37; O, 25.29
|
|
| CAS # |
117048-59-6
|
|
| Related CAS # |
168555-66-6; 222030-63-9; 117048-59-6
|
|
| PubChem CID |
5351344
|
|
| Appearance |
White to light yellow solid powder
|
|
| Density |
1.2±0.1 g/cm3
|
|
| Boiling Point |
490.3±45.0 °C at 760 mmHg
|
|
| Melting Point |
84.5-85.5ºC
|
|
| Flash Point |
250.3±28.7 °C
|
|
| Vapour Pressure |
0.0±1.3 mmHg at 25°C
|
|
| Index of Refraction |
1.607
|
|
| LogP |
3.57
|
|
| Hydrogen Bond Donor Count |
1
|
|
| Hydrogen Bond Acceptor Count |
5
|
|
| Rotatable Bond Count |
6
|
|
| Heavy Atom Count |
23
|
|
| Complexity |
358
|
|
| Defined Atom Stereocenter Count |
0
|
|
| SMILES |
COC1=C(C=C(C=C1)/C=C\C2=CC(=C(C(=C2)OC)OC)OC)O
|
|
| InChi Key |
HVXBOLULGPECHP-WAYWQWQTSA-N
|
|
| InChi Code |
InChI=1S/C18H20O5/c1-20-15-8-7-12(9-14(15)19)5-6-13-10-16(21-2)18(23-4)17(11-13)22-3/h5-11,19H,1-4H3/b6-5-
|
|
| Chemical Name |
2-methoxy-5-[(Z)-2-(3,4,5-trimethoxyphenyl)ethenyl]phenol
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 3 mg/mL (9.48 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 30.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 3 mg/mL (9.48 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 30.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. View More
Solubility in Formulation 3: 5% DMSO +50% PEG 300 +ddH2O: 30mg/mL |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1611 mL | 15.8053 mL | 31.6106 mL | |
| 5 mM | 0.6322 mL | 3.1611 mL | 6.3221 mL | |
| 10 mM | 0.3161 mL | 1.5805 mL | 3.1611 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
|
|---|
 |