
| Size | Price | Stock | Qty |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
Purity: ≥98%
WAY-100635 maleate (WAY 100635; WAY100635), the maleate salt of WAY-100635, is a novel, potent and selective antagonist of serotonin 5-HT1A receptor with important biological activity. It inhibits 5-HT1A receptor with IC50 of 0.95 nM.
| Targets |
D4 Receptor; sPLA2 ( pIC50 = 8.87 ); sPLA2 ( pA2 = 9.71 )
|
||
|---|---|---|---|
| ln Vitro |
|
||
| ln Vivo |
|
||
| Enzyme Assay |
Screening assays[6]
For the initial screens by the NIMH-PDSP at a large number of cloned receptors and transporters (for details, see Roth et al. (2002) and Shapiro et al. (2003)), 10 μM WAY-100635 was used. Where significant inhibition was measured (>50% inhibition with quadruplicate determinations), K i determinations were performed with 6–10 concentrations of unlabelled ligand, and data were analyzed with GraphPad Prism. Saturation binding experiments[6] Whole cell pellets were collected by scraping cells in media, followed by centrifugation at 1,000×g for 10 min and aspirating media. Pellets were then resuspended in ice-cold standard binding buffer (SBB: 50 mM Tris–HCl, pH 7.4, 10 mM MgCl2 and 0.1 mM EDTA), aliquoted, centrifuged at 14,000×g for 20 min at 4°C to pellet the membrane fraction, aspirated, and stored at −80°C for future use.[6] 5-HT1A pellets were washed by resuspending in ice-cold SBB and centrifuged at 14,000×g for 15 min at 4°C, and the buffer was aspirated. hD4.2 pellets were similarly washed but in ice-cold dopamine agonist binding buffer (DABB: 50 mM Tris–HCl pH 7.4, 5 mM KCl, 2 mM MgCl2, 2 mM CaCl2). Washed 5-HT1A membranes were Dounce-homogenized in room temperature SSB and incubated with 12 concentrations of [3H]WAY-100635 ranging from 0.004 to 2.3 nM in the absence and presence of 10 μM 5-HT to determine total and nonspecific binding, respectively. Likewise, washed hD4.2 membranes were Dounce-homogenized in room temperature DABB and incubated with 12 concentrations of [3H]WAY-100635 ranging from 0.004 to 13.4 nM in the absence and presence of 10 μM chlorpromazine to determine total and nonspecific binding, respectively. After 2 h at room temperature, reactions were terminated by rapid filtration onto cold 0.3% PEI presoaked filters. The filters were then washed three times in 4°C 50 mM Tris–HCl, pH 6.9. Filtered material was then transferred to scintillation vials mixed with 4 ml of Ecoscint-A scintillation fluid (National Diagnostic; Atlanta, GA, USA) and counted on a Beckman LS6500 scintillation counter.[6] Radioligand binding assay[6] Cells were grown to confluence on 20-cm plates. The growth medium was decanted and replaced with 10-ml ice-cold lysis buffer (1 mM HEPES, pH 7.4, and 2 mM EDTA). After 10 min, cells were scraped from the plate and centrifuged at 30,000×g and 4°C for 20 min. The resulting pellet was resuspended in 4 ml receptor binding buffer (50 mM Tris–HCl, pH 7.4, and 4 mM MgCl) using a Kinematica homogenizer at a setting of 6 for 5 s, before 1.0 ml aliquots were centrifuged again at 13,000×g for 10 min. The pellets were stored at −80°C until use.[6] The pellets were then resuspended for use by trituration in receptor binding buffer (50 μg protein/100 μl) and added in duplicate to assay tubes containing 0.1–0.2 nM [3H]spiperone and appropriate drugs. Nonspecific binding was determined using (+)-butaclamol (5 μM). Assay tubes were incubated at 37°C for 30 min before filtration, as described for cAMP binding assays. Filter plates were dried, and 30 μl of Packard Microscint-O scintillation fluid was added to each well. Radioactivity per well was determined using a Packard TopCount scintillation counter.[6] Radioligand binding assays were also performed to investigate the effect of 100 μM guanylyl-5′-imidodiphosphate (Gpp-[NH]p) on agonist binding. These experiments were performed using HEK-hD4.4 membranes in a modified receptor binding buffer (50 mM Tris–HCl, pH 7.4, 4 mM MgCl, and 120 mM NaCl). |
||
| Cell Assay |
Extracellular recordings are performed using glass microelectrodes that have been loaded with 2 M NaC1 (12 MΩ–15 MΩ). Two or three-millisecond biphasic action potentials, slow (0.5 Hz - 2.0 Hz) discharge patterns, and regular discharge patterns are characteristics that distinguish cells as 5-HT neurons. The alpha-l adrenergic agonist phenylephrine (3 μM) is added to the superfusing ACSF to cause firing in the otherwise silent neurons. Prior to applying the various medications, baseline activity is tracked for at least ten minutes. Precise action potentials coupled to an A/D converter and a PC drive individual action potentials that drive an oscilloscope, an electronic ratemeter, and a high-input impedance amplifier. The integrated firing rate is measured, calculated, and shown on a chart recorder as successive 10-sec samples using specialized software. Agonists' effects are assessed by comparing the mean discharge frequency recorded during the two minutes prior to WAY 100635 application with the frequency recorded at the peak of the drug's action, which is typically two to five minutes after application starts. The effect of the agonist is contrasted with the baseline firing rate and frequency observed during the antagonist's single superfusion when the agonists are applied in the presence of the antagonist. Before retesting the agonists' action, the antagonist is given ten to twenty-five minutes to acclimate.
|
||
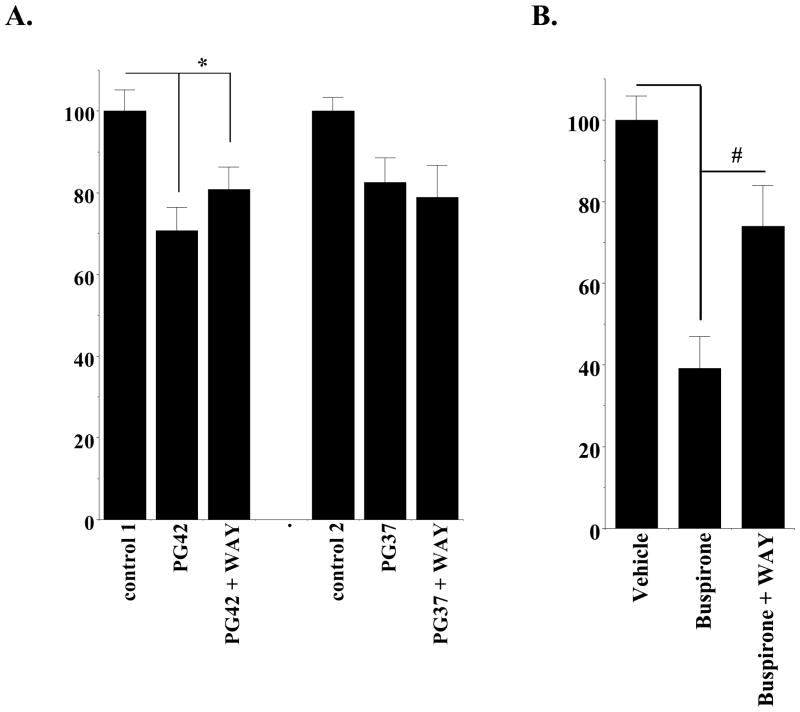
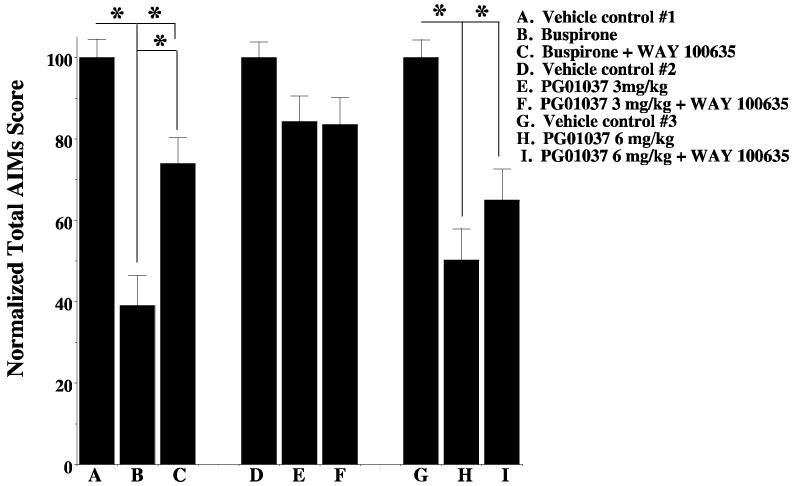
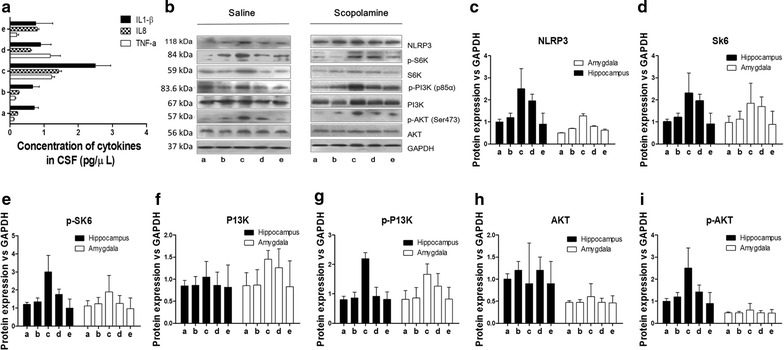
| Animal Protocol |
|
||
| References | |||
| Additional Infomation |
The aim of the present study was to examine the effects of N-(2-(4-2-methoxphenyl)-1-piperazinyl)ethyl)-N-(2-pyridnyl) cyclohexane carboxamide (WAY 100635) on 5-HT1A receptor-mediated responses in the dorsal raphe nucleus (DRN) and the CA1 hippocampal region. In DRN slices superfused with WAY 100635 (10 nM), the majority of putative 5-HT neurons increased their firing rate (13 +/- 2% of baseline rate). In addition, WAY 100635 completely prevented the decrease in firing rate produced by 5-HT (3-15 microM), 8-OH-DPAT (10 nM), 5-carboxamidotryptamine (20 nM) and lesopitron (100 nM). The antagonism exerted by WAY 100635 (IC50 = 0.95 +/- 0.12 nM against 15 microM 5-HT) was fully surmounted by increasing the concentration of 5-HT to 300 microM. In hippocampal slices, WAY 100635 (0.5-10 nM) did not alter the resting membrane potential or the membrane input resistance of intracellularly recorded CA1 pyramidal cells. However, WAY 100635 completely prevented (IC50 = 0.9-1.7 nM) the hyperpolarization and the decrease in membrane input resistance produced by 5-HT (15-30 microM) and by 5-carboxamidotryptamine (50-300 nM). In contrast, WAY 100635 affected neither the block of action potential frequency adaptation and slow afterhyperpolarization produced by 5-HT (15 microM) nor the hyperpolarization and decrease in membrane input resistance evoked by bath application of GABA(B) receptor agonist baclofen (10 microM). The cumulative concentration-hyperpolarization curve for 5-carboxamidotryptamine (3 nM-10 microM) was shifted to the right by WAY 100635 (apparent Kb = 0.23 +/- 0.07 nM), and the latter drug also reduced the maximal response to the agonist. These data show the WAY 100635 is a potent antagonist at 5-HT1A receptors, both in the DRN and in the CA1 region of the hippocampus. The antagonism is apparently competitive in the DRN and partly noncompetitive in the hippocampus. Kinetic characteristics of the antagonist-receptor interactions might account for these regional differences.[1]
Rationale and objectives: WAY-100635 is a prototypical 5-HT1A receptor antagonist and has been used widely as a pharmacological probe to investigate the distribution and function of 5-HT1A receptors. Results from our studies suggested that WAY-100635 was potently inducing effects unrelated to its 5-HT1A receptor affinity. In the present work, we evaluated the in vitro pharmacology of this compound at two D2-like receptor subtypes. Method: The functional properties and binding affinities of WAY-100635 were evaluated in HEK 293 cells stably expressing dopamine D2L or D4.4 receptors. Results: Initial screens performed by the NIMH Psychoactive Drug Screening Program indicated that WAY-100635 displayed 940, 370, and 16 nM binding affinities at D2L, D3, and D4.2 receptors, respectively. Subsequent saturation analyses demonstrated that the Kd of [3H]WAY-100635 at D4.2 receptors was 2.4 nM, only tenfold higher than 5-HT1A. WAY-100635 and its major metabolite, WAY-100634, were potent agonists in HEK-D4.4 cells (EC50=9.7+/-2.2 and 0.65+/-0.2 nM, respectively). WAY-100635 behaved as a full agonist, and WAY-100634 was a nearly full agonist. In HEK-D2L cells, WAY-100635 weakly antagonized the effects of 300 nM quinpirole. Subsequent radioligand binding studies confirmed that WAY-100635 possesses high affinity for D4.4 receptors but binds weakly to D2L receptors (3.3+/-0.6 and 420+/-11 nM, respectively). Conclusions: This study demonstrates that WAY-100635 is not a "selective" 5-HT1A receptor antagonist, as previously reported, and conclusions drawn from studies that employed WAY-100635 as a selective 5-HT1A antagonist may need to be reevaluated. [6] The distribution of 5-HT1A receptors was examined in the post-mortem human brain using whole hemisphere autoradiography and the selective 5-HT1A receptor antagonist [3H]WAY-100635 ([O-methyl-3H]-N-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2- pyridinyl)cyclohexanecarboxamide trihydrochloride). The autoradiograms showed very dense binding to hippocampus, raphe nuclei and neocortex. The labeling in neocortex was slightly lower than in the hippocampus and was mainly at superficial layers, although a faintly labeled band could be seen in deeper neocortical layers. Other regions, such as the amygdala, septum and claustrum, showed low densities caudatus and putamen, in cerebellum or in structures of the brain stem except in the raphe nuclei. The labeling of human 5-HT1A receptors with [3H]WAY-100635 was antagonised by the addition of 5-HT1A receptor ligands, 5-HT, buspirone, pindolol or 8-OH-DPAT (10 microM), leaving a very low background of non-specific binding. Saturation analysis of semiquantitative data from several human regions indicated that [3H]WAY-100635 has a Kd of approximately 2.5 nM. The selective labeling of 5-HT1A receptors with [3H]WAY-100635 clearly show that this compound is useful for further studies of the human 5-HT1a receptor subtype in vitro [11C]WAY-100635 is used for the characterization of 5-HT1A receptors with positron emission tomography (PET). WAY-100635 was also radiolabeled with the short-lived positron-emitting radionuclide carbon-11 (t1/2 = 20 min) and used for in vitro autoradiography on human whole hemisphere cryosections. [11C]WAY-100635 gave images qualitatively similar to those of [3H]WAY-100635, although with a lower resolution. Thus, the hippocampal formation was densely labeled, with lower density in the neocortex. Buspirone, pindolol or 8-OH-DPAT (10 microM), blocked all binding of [11C]WAY-100635. The in vitro autoradiography of the distribution of 5-HT1A receptors obtained with radiolabeled WAY-100635 provide detailed qualitative and quantitative information on the distribution of 5-HT1A-receptors in the human brain. Moreover, the studies give reference information for the interpretation of previous initial results at much lower resolution in humans with PET and [11C]Way-100635. These data provide a strong basis for expecting [11C]WAY-100635 to behave as a highly selective radioligand in vivo.[3] |
| Molecular Formula |
C29H38N4O6
|
|
|---|---|---|
| Molecular Weight |
538.64
|
|
| Exact Mass |
538.279
|
|
| Elemental Analysis |
C, 64.67; H, 7.11; N, 10.40; O, 17.82
|
|
| CAS # |
1092679-51-0
|
|
| Related CAS # |
WAY-100635; 162760-96-5
|
|
| PubChem CID |
11957721
|
|
| Appearance |
White to off-white solid powder
|
|
| LogP |
3.54
|
|
| Hydrogen Bond Donor Count |
2
|
|
| Hydrogen Bond Acceptor Count |
9
|
|
| Rotatable Bond Count |
9
|
|
| Heavy Atom Count |
39
|
|
| Complexity |
665
|
|
| Defined Atom Stereocenter Count |
0
|
|
| SMILES |
O=C(O)/C=C\C(O)=O.O=C(N(C1=NC=CC=C1)CCN2CCN(CC2)C3=CC=CC=C3OC)C4CCCCC4.[1:1]
|
|
| InChi Key |
XIGAHNVCEFUYOV-BTJKTKAUSA-N
|
|
| InChi Code |
InChI=1S/C25H34N4O2.C4H4O4/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21;5-3(6)1-2-4(7)8/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3;1-2H,(H,5,6)(H,7,8)/b;2-1-
|
|
| Chemical Name |
(Z)-but-2-enedioic acid;N-[2-[4-(2-methoxyphenyl)piperazin-1-yl]ethyl]-N-pyridin-2-ylcyclohexanecarboxamide
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: 25 mg/mL (46.41 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with sonication (<60°C).
(Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8565 mL | 9.2826 mL | 18.5653 mL | |
| 5 mM | 0.3713 mL | 1.8565 mL | 3.7131 mL | |
| 10 mM | 0.1857 mL | 0.9283 mL | 1.8565 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
 |
|---|
 |
 |