
| Size | Price | Stock | Qty |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
Purity: ≥98%
| Targets |
SRMS (IC50 = 18 nM); ACK1 (IC50 = 19 nM); B-Raf (V600E) (IC50 = 48 nM); MAP4K5 (KHS1) (IC50 = 51 nM); C-Raf (IC50 = 48 nM)
|
|---|---|
| ln Vitro |
Vemurafenib (PLX4032) specifically inhibits the RAF/MEK/ERK pathway in BRAF mutant cells[1]. In 17 melanoma cell lines, RG7204 is a potent inhibitor of proliferation in those that express RAFV600E but not BRAFWT. High concentrations of vemurafenib (RG7204) cause MEK and ERK phosphorylation in CHL-1 cells[2]. Resistance to PLX4032 can be brought on by EGFR expression in melanoma cells that is ectopically expressed[3].
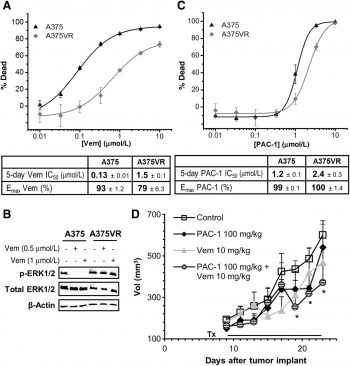
The BRAF(V600E) mutation is common in several human cancers, especially melanoma. RG7204 (PLX4032) is a small-molecule inhibitor of BRAF(V600E) kinase activity that is in phase II and phase III clinical testing. Here, we report a preclinical characterization of the antitumor activity of RG7204 using established in vitro and in vivo models of malignant melanoma. RG7204 potently inhibited proliferation and mitogen-activated protein/extracellular signal-regulated kinase (ERK) kinase and ERK phosphorylation in a panel of tumor cell lines, including melanoma cell lines expressing BRAF(V600E) or other mutant BRAF proteins altered at codon 600[2]. |
| ln Vivo |
Vemurafenib (PLX4032, 20, 25, 75 mg/kg, p.o.) inhibits tumor growth in a dose-dependent manner, with higher exposures leading to tumor regression in xenografts harboring the BRAF mutation[1]. In mice bearing LOX tumor xenografts, RG7204 (12.5, 25, and 75 mg/kg, p.o.) significantly inhibits tumor growth and causes tumor regression[2].
In several tumor xenograft models of BRAF(V600E)-expressing melanoma, researchers found that RG7204 treatment caused partial or complete tumor regressions and improved animal survival, in a dose-dependent manner. There was no toxicity observed in any dose group in any of the in vivo models tested. |
| Enzyme Assay |
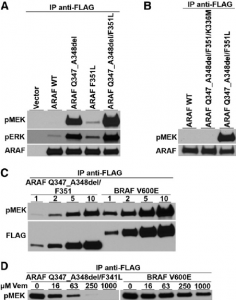
PLX4032 kinase selectivity As mentioned in the text, when the kinase selectivity panel was expanded to over 200 members, several additional kinases were found to be sensitive to PLX4032. Most of these kinases were assayed at a lower ATP concentration (10 μM for the counter-screens versus 100 μM for the RAF kinases); since PLX4032 is a competitive inhibitor assay at the lower ATP concentration results in lower IC50 values. In a panel of over 150 chemical analogs of PLX4032, there was good correlation between biochemical potency for B-RAFV600E and cellular activity against B-RAF-mutant cells. This correlation did not depend on the relative potency against B-RAFV600E and wild type B-RAF. Therefore, we believe that efficacy in melanoma primarily derives from inhibition of mutant B-RAF; future studies may explore the role of off-targets in other indications[1].
When PLX4032 was co-crystallized with B-RAFV600E, two unique molecules of the kinase domain in the asymmetric unit adopt a side- to-side dimer configuration as observed in previous RAF crystal structures. Previously, PLX4720 was co-crystallized with wild type B-RAF, and the protomer with only partial ligand occupancy (apo) adopts a DFG-out conformation representing the inactive state of the kinase. However, the apo-protomer in the PLX4032 co-structure with B-RAFV600E displays the DFG-in conformation with the activation loop locked away from the ATP-binding site by a salt-bridge between Glu600 and Lys507 (Figure 1D). Subsequent analysis of the structure of PLX4720 co-crystallized with B-RAFV600E revealed that the apo-protomer displays the DFG-in conformation, suggesting that this property is determined by the mutation. It is interesting to speculate that the conformation of the apo-protomer may determine the paradoxical activation described in the main text. The conformational difference captured by the crystal structures (Figure 1C) suggests that, although wild-type B-RAF is in a dynamic equilibrium between the active (DFG-in) and inactive (DFG-out) configurations, oncogenic BRAF mutations such as V600E induce constitutive kinase activity by shifting the equilibrium toward the active (DFG-in) configuration. We believe that selective binding to the DFG-in conformation may contribute to a wide safety margin because such inhibitors would suppress the tumor growth but spare the important biological functions mediated by wild-type B-RAF kinases[1]. |
| Cell Assay |
Briefly, cells are plated in 96-well microtiter plates with a volume of 180 μL at a density of 1,000 to 5,000 cells per well. Vemurafenib (RG7204) is prepared for the assay in media containing 1% DMSO at 10 times the final assay concentration. 20 μL of the appropriate dilution are added to plates in duplicate twenty-four hours after cell plating. Six days after the cells are plated, the plates are tested for proliferation in accordance with the procedure.[2]
For sample preparation from cell lines, the cells were seeded at appropriate density (70–75% confluent) in six-well plates 1 day before compound treatment. Upon compound treatment at various drug concentrations for 2 hours at 37°C, the cells were harvested and lysed immediately. For sample preparation from tumor xenografts, tumors were harvested at the indicated time points and stored at −80°C. Protein was extracted by homogenization in the presence of 2 to 5 mL lysis buffer. After incubation on ice for 20 to 30 minutes, the lysates were centrifuged at 14,000 rpm for 15 minutes. The protein concentrations of the lysates were determined. Equal amounts of total protein for cell lysates and for tumor lysates were resolved on 4% to 12% NuPage gradient polyacrylamide gels and blotted with the indicated antibodies. The chemiluminescent signal was generated with Enhanced Chemiluminescence Plus Western Blotting Detection Reagents and detected with a Fujifilm LAS-3000 imager. The densitometric quantitation of specific bands was determined using the Multi Gauge Software[2]. |
| Animal Protocol |
Athymic nude mice have a lifespan of 13 to 14 weeks and weigh between 23 and 25 g. 2×106 cells in 0.2 mL of PBS are injected subcutaneously into the right lateral flank for the LOX xenografts. In an aqueous vehicle containing 2% Klucel LF and pH 4-adjusted with diluted HCl, vemurafenib (RG7204), formulated as MBP, is suspended at the required concentration as needed for each dose group. There are 250-mg capsules of NSC 362856. Opened capsules are collected into a single bulk supply. NSC 362856 is first dissolved in 100% DMSO, then the DMSO is diluted with saline to create a final milky white suspension in 10% DMSO/90% saline (pH 3.4), which is the stock dosing material.
|
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
Vemurafenib is well absorbed after oral administration. Peak concentrations are reached in 3 hours when an oral dose of 960 mg twice daily for 15 days has been given to patients. In the same conditions, Vemurafenib presents a Cmax of 62 mcg/ml and AUC of 601 mcg h/ml. It is unknown how food affects the absorption of vemurafenib. It presents an accumulation ratio of 7.36 after repeating doses of 960 mg Analysis showed that 94% of administered Vemurafenib is excreted via feces and 1% is excreted by urine. The estimation of the volume of distribution for Vemurafenib is 106 L. The total body clearance is 31 L/day. Following oral administration of (14)C-vemurafenib 960 mg in the tablet formulation, plasma samples were analyzed over 48 hours for vemurafenib and its metabolites. Mean data showed that vemurafenib and its metabolites represented 95% and 5% of the components in plasma, respectively. Vemurafenib is highly bound (> 99%) to human albumin and alpha-1 acid glycoprotein plasma proteins. The population apparent volume of distribution for vemurafenib in metastatic melanoma patients is estimated to be 106 L (with 66% inter-patient variability). The bioavailability of vemurafenib has not been determined. Following oral administration of vemurafenib at 960 mg twice daily for 15 days to patients with metastatic melanoma, the median Tmax was approximately 3 hours. Following 15 days of dosing at 960 mg twice daily, the mean (+ or - SD) Cmax and AUC0-12 were 62 ug/mL + or - 17 and 601 + or - 170 ug*h/mL, respectively. The median accumulation ratio estimate from the population pharmacokinetic analysis for the twice daily regimen is 7.36, with steady state achieved at approximately 15 to 22 days following dosing at 960 mg twice daily. At steady state, the mean vemurafenib exposure in plasma is stable (concentrations before and 2-4 hours after the morning dose) as indicated by the mean ratio of 1.13. The potential effect of food on vemurafenib absorption has not been studied. In clinical trials, vemurafenib was administered without regard to food. Following oral administration of (14)C-vemurafenib 960 mg in the tablet formulation, approximately 94% of the radioactive dose was recovered in feces and approximately 1% was recovered in the urine. The population apparent clearance of vemurafenib in patients with metastatic melanoma is estimated to be 31 L/day (with 32% inter-patient variability). For more Absorption, Distribution and Excretion (Complete) data for Vemurafenib (6 total), please visit the HSDB record page. Metabolism / Metabolites Vemurafenib is metabolized by CYP3A4 and the metabolites make up 5% of the components in plasma. The parent compound makes up for the remaining 95%. The results from in vitro studies indicate that CYP3A4 was the major enzyme responsible in the metabolism of vemurafenib. The formation of mono-hydroxyl metabolites were inhibited for approximately 82% using the CYP inhibitor ketoconazole. No significant inhibition in the metabolism was observed in human liver microsomes in the presence of quinidine (CYP2D6 inhibitor), sulfaphenazole (CYP2C9 inhibitor), tranylcypromine (CYP2A6 inhibitor) and (-)-N-3-benzyl-phenobarbital (CYP2C19 inhibitor). In addition, CYP3A4 was responsible for the formation of the mono-hydroxylation metabolites. In vitro metabolism was analyzed for rat, mouse, dog, cynomolgus and human. The metabolism of vemurafenib was investigated both in vitro using microsomes and hepatocytes of various species and in vivo in rat, dog and human. In vitro analysis of vemurafenib metabolism in liver hepatocytes at the concentration of 10 uM, humans, dogs, and cynomolgus monkeys did not metabolize vemurafenib extensively (unchanged vemurafenib > or = 89%). In study /of patients/, identification of vemurafenib and metabolites in plasma, feces and urine was made for the first 96 hr, with a total collection period of 432 hrs (18 days). Mean data from the 7 patients indicated that over the period investigated (0 to 96 hours), potential metabolites each accounted for < 0.5% of the total administered dose in urine and .6% of the total administered dose in feces. In pooled fecal samples up to 48 hours post post-dose, parent compound accounted for at least 94% of total radioactivity (37% of the dose). In fecal samples taken 48-96 hr post-dose, the amount of metabolites increased, with M6, M3, and M8 representing approximately 19%, 14% and 12%, of the total chromatographic peak area, respectively (mean values) or 3%, 5% and 4% of the dose, respectively. Over the 0-96 hr collection period, potential metabolites M3 (mono-hydroxy) and M6 (glucosylation) each accounted for <0.5% of the total administered dose in urine. Vemurafenib accounted for approximately 1% of the total dose in urine. Biological Half-Life The elimination half-life of Vemurafenib is estimated to be 57 hours (range of 30-120 hours). Single dose studies to determine pharmacokinetics were conducted in mouse, rat, rabbit, dog and monkey. In all pre-clinical species, half-lifes were between 2 and 5 hours ... . Only after intraperitoneal (IP) administration in mice, the half-life was much longer (20.6 h). Compared with other species, rabbits showed higher plasma exposure levels with a longer mean terminal half-life between 12 and 18 hours. The median of the individual elimination half-life estimates for vemurafenib is 57 hours (the 5th and 95th percentile range is 30 to 120 hours). |
| Toxicity/Toxicokinetics |
Hepatotoxicity
In large clinical trials of vemurafenib, abnormalities in routine liver tests were common and serum aminotransferase elevations occurred in up to one third of patients. ALT and AST values greater than 5 times the upper limit of normal (ULN) occurred in 3% of patients, and rare instances of clinically apparent liver injury were reported, but the clinical features of the injury have not been described. The onset of liver test abnormalities was typically within 3 to 6 weeks of starting vemurafenib, and the abnormalities resolved rapidly either spontaneously or with temporary drug discontinuation. Vermurafenib has also been linked to instances of drug related rash with eosinophilia and systemic manifestations (DRESS) as well as Stevens Johnson syndrome, both of which can be accompanied by liver dysfunction and in some cases jaundice with clinically apparent liver injury. Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury. Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of vemurafenib during breastfeeding. Because vemurafenib is more than 99% bound to plasma proteins, the amount in milk is likely to be low. However, its half-life is 57 hours and it might accumulate in the infant. The manufacturer recommends that breastfeeding be discontinued during vemurafenib therapy and for 2 weeks after the final dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Vemurafenib highly binds to plasma proteins where >99% of the administered dose will be found protein bound to serum albumin and alpha-1 acid glycoprotein. Interactions Concomitant use of vemurafenib with drugs known to prolong the QT interval, including class Ia (e.g., quinidine, procainamide) and class III (e.g., amiodarone, sotalol) antiarrhythmic agents, some antipsychotic agents (e.g., chlorpromazine, thioridazine, haloperidol, asenapine, olanzapine, paliperidone, pimozide, quetiapine, ziprasidone), some antibiotics (e.g., gatifloxacin, moxifloxacin), and tetrabenazine is not recommended by the manufacturer. Concomitant use of vemurafenib with CYP2C9 substrates may result in increased plasma concentrations of the CYP2C9 substrate and possible toxicity. When the CYP2C9 substrate warfarin was administered concomitantly with vemurafenib, the systemic exposure of S-warfarin increased by 18%. Vemurafenib and warfarin should be used concomitantly with caution and additional monitoring of the international normalized ratio (INR) should be considered. Concomitant use of vemurafenib with CYP3A4 substrates may result in decreased plasma concentrations of the CYP3A4 substrate and possible decreased efficacy. When the CYP3A4 substrate midazolam was administered concomitantly with vemurafenib, the systemic exposure of midazolam decreased by 39%. Concomitant use of vemurafenib with CYP3A4 substrates that have a narrow therapeutic index should be avoided. Concomitant use of vemurafenib with CYP2D6 substrates may result in increased plasma concentrations of the CYP2D6 substrate and possible toxicity. When the CYP2D6 substrate dextromethorphan was administered concomitantly with vemurafenib, the systemic exposure of dextromethorphan increased by 47%. Concomitant use of vemurafenib with CYP2D6 substrates that have a narrow therapeutic index should be avoided. If concomitant use cannot be avoided, dosage reduction of the CYP2D6 substrate should be considered, and the drugs should be used concomitantly with caution. For more Interactions (Complete) data for Vemurafenib (9 total), please visit the HSDB record page. |
| References |
|
| Additional Infomation |
Therapeutic Uses
Vemurafenib is used for the treatment of unresectable or metastatic melanoma with BRAF V600E mutation. Vemurafenib is designated an orphan drug by the US Food and Drug Administration (FDA) for the treatment of this cancer. An FDA-approved diagnostic test (e.g., cobas 4800 BRAF V600 Mutation Test) is required to confirm the presence of the BRAF V600E mutation prior to initiation of therapy. /Included in US product label/ Zelboraf is not recommended for use in patients with wild-type BRAF melanoma. Drug Warnings Serious hypersensitivity reactions (e.g., anaphylaxis, generalized rash and erythema, hypotension) have been reported in patients receiving vemurafenib. Vemurafenib should be permanently discontinued in patients who experience a severe hypersensitivity reaction. Photosensitivity reactions (mild to severe) have been reported in 33-49% of patients receiving vemurafenib in clinical trials. If intolerable grade 2 (i.e., tender erythema covering 10-30% of body surface area) or greater reaction occurs, the dosage of vemurafenib should be reduced. Vemurafenib prolongs the QT interval in a concentration-dependent manner. In a multicenter, open-label, phase 2 study, QT interval prolongation was evaluated in patients with BRAF V600E mutation-positive, metastatic melanoma who were receiving vemurafenib (960 mg twice daily). A maximum mean corrected QT (QTc) interval change from baseline of 12.8 msec during the first month of treatment and 15.1 msec during the first 6 months of treatment was observed in these patients. The manufacturer does not recommend initiation of vemurafenib in patients with electrolyte abnormalities unresponsive to corrective measures or congenital long QT syndrome. In addition, concomitant use of vemurafenib with drugs known to prolong the QT interval (e.g., class Ia and III antiarrhythmic agents) is not recommended. ECGs and serum electrolyte concentrations, including concentrations of potassium, magnesium, and calcium, should be obtained prior to initiation of therapy or following dosage modification, and monitored 15 days following initiation of therapy, then monthly for the first 3 months of treatment, and then every 3 months thereafter or more often as clinically indicated. Interruption or discontinuance of vemurafenib may be necessary if increases in the QTc interval occur during therapy with the drug. Severe skin reactions (e.g., Stevens-Johnson syndrome, toxic epidermal necrolysis) have been reported with vemurafenib. If severe skin reactions occur, vemurafenib therapy should be permanently discontinued. For more Drug Warnings (Complete) data for Vemurafenib (18 total), please visit the HSDB record page. Pharmacodynamics BRAF activation results in cell growth, proliferation, and metastasis. BRAF is an intermediary molecule in MAPK whose activation depends on ERK activation, elevation of cyclin D1 and cellular proliferation. The mutation V600E produces a constitutively form of BRAF. Vemurafenib has been shown to reduce all activation markers related to BRAF; in clinical trials, vemurafenib treatment showed a reduction of cytoplasmic phosphorylated ERK and a cell proliferation driven by Ki-67. Studies also reported decrease in MAPK-related metabolic activity. All the different reports indicate thet Vemurafenib generates an almost complete inhibition of the MAPK pathway. |
| Molecular Formula |
C23H18CLF2N3O3S
|
|---|---|
| Molecular Weight |
489.92
|
| Exact Mass |
489.072
|
| Elemental Analysis |
C, 56.39; H, 3.70; Cl, 7.24; F, 7.76; N, 8.58; O, 9.80; S, 6.54
|
| CAS # |
918504-65-1
|
| Related CAS # |
Vemurafenib-d5;1365986-90-8;Vemurafenib-d7;1365986-73-7; 918505-61-0 (analog); 918504-65-1
|
| PubChem CID |
42611257
|
| Appearance |
White to off-white crystalline solid
|
| Density |
1.5±0.1 g/cm3
|
| Boiling Point |
711.4±70.0 °C at 760 mmHg
|
| Melting Point |
260-262 °C
|
| Flash Point |
384.0±35.7 °C
|
| Vapour Pressure |
0.0±2.3 mmHg at 25°C
|
| Index of Refraction |
1.653
|
| LogP |
4.26
|
| Hydrogen Bond Donor Count |
2
|
| Hydrogen Bond Acceptor Count |
7
|
| Rotatable Bond Count |
7
|
| Heavy Atom Count |
33
|
| Complexity |
790
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
O=C(C1C(F)=C(NS(CCC)(=O)=O)C=CC=1F)C1C2C(=NC=C(C3C=CC(Cl)=CC=3)C=2)NC=1
|
| InChi Key |
GPXBXXGIAQBQNI-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C23H18ClF2N3O3S/c1-2-9-33(31,32)29-19-8-7-18(25)20(21(19)26)22(30)17-12-28-23-16(17)10-14(11-27-23)13-3-5-15(24)6-4-13/h3-8,10-12,29H,2,9H2,1H3,(H,27,28)
|
| Chemical Name |
N-[3-[5-(4-chlorophenyl)-1H-pyrrolo[2,3-b]pyridine-3-carbonyl]-2,4-difluorophenyl]propane-1-sulfonamide
|
| Synonyms |
Vemurafenib; RO5185426; RG7204; PLX 4032; RG 7204; RO 5185426; RG-7204; RO5185426; PLX4032; PLX-4032; trade name: Zelboraf; N-(3-(5-(4-Chlorophenyl)-1H-pyrrolo[2,3-b]pyridine-3-carbonyl)-2,4-difluorophenyl)propane-1-sulfonamide;
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (4.25 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (4.25 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. View More
Solubility in Formulation 3: 4% DMSO +30% PEG 300 +5% Tween 80 +ddH2O: 5mg/mL Solubility in Formulation 4: 3.33 mg/mL (6.80 mM) in 1.5% CMC-Na/saline water (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0411 mL | 10.2057 mL | 20.4115 mL | |
| 5 mM | 0.4082 mL | 2.0411 mL | 4.0823 mL | |
| 10 mM | 0.2041 mL | 1.0206 mL | 2.0411 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
A Study in Patients Previously Enrolled in a Genentech and/or F. Hoffmann-La Roche Ltd Sponsored Atezolizumab Study
CTID: NCT03768063
Phase: Phase 3 Status: Recruiting
Date: 2024-11-20
Mol Cancer Ther; 15(8); 1859–69, 2016 |
 |
 |