
| Size | Price | Stock | Qty |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g | |||
| 5g | |||
| Other Sizes |
Purity: ≥98%
Terazosin HCl dihydrate (Hytrin; A-45975; A45975), the hydrochloride salt and dihydrated form of terazosin, is a potent and selective α1-adrenoceptor antagonist with antihypertensive effects. It has been applied to treat benign prostatic hyperplasia, or BPH (enlargening of the prostate) symptoms. In PC-3 and human benign prostatic cells, terazosin causes cytotoxicity with an IC50 greater than 100 μM. In cultured human umbilical vein endothelial cells, terazosin also effectively inhibited tube formation and vascular endothelial growth factor-induced proliferation (IC50 9.9 and 6.8 μM, respectively).
| Targets |
α-adrenergic receptor
|
||
|---|---|---|---|
| ln Vitro |
|
||
| ln Vivo |
|
||
| Cell Assay |
The present study employed multiple identification techniques to ascertain the mode of action of the cytotoxic effect. Use of terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling allows for the in situ detection of apoptotic cells. Data indicate that PC-3 cells treated with 100 μM terazosin for 12 hours showed a positive response.
PC-3 cells and primary cultures of human benign prostatic cells were used in this study. The cytotoxic effect was examined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and lactate dehydrogenase release reaction. The in vivo angiogenic effect was determined in nude mice models, followed by histological examination and quantification by the hemoglobin detection assay. In vitro determination of cell migration, proliferation and tube formation was performed in cultured human umbilical vein endothelial cells. RESULTS terazosin induced cytotoxicity in PC-3 and human benign prostatic cells with an IC50 of more than 100 microM. The positive terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling and lactate dehydrogenase release reaction was associated with terazosin induced cytotoxicity, indicating apoptotic and necrotic cell death. Furthermore, cytotoxicity due to terazosin action was not a common characteristic of a quinazoline based structure. Terazosin significantly inhibited vascular endothelial growth factor induced angiogenesis in nude mice with an IC50 of 7.9 microM., showing that it had a more potent anti-angiogenic than cytotoxic effect. Terazosin also effectively inhibited vascular endothelial growth factor induced proliferation and tube formation in cultured human umbilical vein endothelial cells (IC50 9.9 and 6.8 microM., respectively). Conclusions: Together our data suggest that terazosin shows direct anti-angiogenic activity through the inhibition of proliferation and tube formation in endothelial cells. This action may partly explain the in vivo antitumor potential of terazosin[4]. |
||
| Animal Protocol |
|
||
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
Approximately 90%. Approximately 10% of the oral dose is excreted unchanged in the urine and approximately 20% is excreted in the feces. 40% of the total dose is eliminated in urine and 60% of the total dose is eliminated in the feces. 25L to 30L. Plasma clearance is 80mL/min and renal clearance is 10mL/min. Metabolism / Metabolites The majority of terazosin is hepatically metabolized. The metabolites recovered include 6-O-demethyl terazosin, 7-O-methyl terazosin, a piperozine derivative, and a diamine derivative. Hepatic. One of the four metabolites identified (piperazine derivative of terazosin) has antihypertensive activity. Route of Elimination: Approximately 10% of an orally administered dose is excreted as parent drug in the urine and approximately 20% is excreted in the feces. Half Life: 12 hours Biological Half-Life Terazosin has a mean half life 12 hours though this can be as high as 14 hours in patients over 70 years and as low as 11.4 hours in patients 20 to 39 years old. |
||
| Toxicity/Toxicokinetics |
Toxicity Summary
Terazosin selectively and competitively inhibits vascular postsynaptic alpha(1)-adrenergic receptors, resulting in peripheral vasodilation and a reduction of vascular resistance and blood pressure. Unlike the nonselective alph-adrenergic blockers phenoxybenzamine and phentolamine, terazosin does not block presynaptic alpha(2)-receptors and, hence, does not cause reflex activation of norepinephrine release to produce reflex tachycardia. Hepatotoxicity Terazosin has been associated with a low rate of serum aminotransferase elevations that in controlled trials was no higher than with placebo therapy. These elevations were transient and did not require dose modification. Instances of serum enzyme elevations, but no instances of clinically apparent acute liver injury with jaundice due to terazosin, have been published. Furthermore, product labels do not include discussion of hepatic toxicity. Cholestatic hepatitis and jaundice have been reported with other alpha-adrenergic blockers. Thus, acute symptomatic liver injury due to terazosin must be exceedingly rare if it occurs at all. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Because no information is available on the use of terazosin during breastfeeding, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information in nursing mothers was not found as of the revision date. However, the pharmacologically similar drug prazosin does not affect serum prolactin concentration in patients with hypertension. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Protein Binding 90-94%. |
||
| References | |||
| Additional Infomation |
Recent evidence from our laboratory has demonstrated that alpha1-adrenoceptor antagonists doxazosin and terazosin induced apoptosis in prostate epithelial and smooth muscle cells in patients with benign prostatic hypertrophy (BPH; J. Urol., 159: 1810-1815, 1998; J. Urol., 161: 2002-2007, 1999). In this study, we investigated the biological action of three alpha1-adrenoceptor antagonists, doxazosin, terazosin, and tamsulosin, against prostate cancer cell growth. The antigrowth effect of the three alpha1-adrenoceptor antagonists was examined in two human prostate cancer cell lines, PC-3 and DU-145, and a prostate smooth muscle cell primary culture, SMC-1, on the basis of: (a) cell viability assay; (b) rate of DNA synthesis; and (c) induction of apoptosis. Our results indicate that treatment of prostate cancer cells with doxazosin or terazosin results in a significant loss of cell viability, via induction of apoptosis in a dose-dependent manner, whereas tamsulosin had no effect on prostate cell growth. Neither doxazosin nor terazosin exerted a significant effect on the rate of cell proliferation in prostate cancer cells. Exposure to phenoxybenzamine, an irreversible inhibitor of alpha1-adrenoceptors, does not abrogate the apoptotic effect of doxazosin or terazosin against human prostate cancer or smooth muscle cells. This suggests that the apoptotic activity of doxazosin and terazosin against prostate cells is independent of their capacity to antagonize alpha1-adrenoceptors. Furthermore, an in vivo efficacy trial demonstrated that doxazosin administration (at tolerated pharmacologically relevant doses) in SCID mice bearing PC-3 prostate cancer xenografts resulted in a significant inhibition of tumor growth. These findings demonstrate the ability of doxazosin and terazosin (but not tamsulosin) to suppress prostate cancer cell growth in vitro and in vivo by inducing apoptosis without affecting cell proliferation. This evidence provides the rationale for targeting both drugs, already in clinical use and with established adverse-effect profiles, against prostatic tumors for the treatment of advanced prostate cancer.[2]
Human ether-a-go-go-related gene (HERG) potassium channels are expressed in multiple tissues including the heart and adenocarcinomas. In cardiomyocytes, HERG encodes the alpha-subunit underlying the rapid component of the delayed rectifier potassium current, I(Kr), and pharmacological reduction of HERG currents may cause acquired long QT syndrome. In addition, HERG currents have been shown to be involved in the regulation of cell proliferation and apoptosis. Selective alpha 1-adrenoceptor antagonists are commonly used in the treatment of hypertension and benign prostatic hyperplasia. Recently, doxazosin has been associated with an increased risk of heart failure. Moreover, quinazoline-derived alpha 1-inhibitors induce apoptosis in cardiomyocytes and prostate tumor cells independently of alpha1-adrenoceptor blockade. To assess the action of the effects of prazosin, doxazosin, and terazosin on HERG currents, we investigated their acute electrophysiological effects on cloned HERG potassium channels heterologously expressed in Xenopus oocytes and HEK 293 cells.Prazosin, doxazosin, and terazosin blocked HERG currents in Xenopus oocytes with IC(50) values of 10.1, 18.2, and 113.2 microM respectively, whereas the IC(50) values for HERG channel inhibition in human HEK 293 cells were 1.57 microM, 585.1 nM, and 17.7 microM. Detailed biophysical studies revealed that inhibition by the prototype alpha 1-blocker prazosin occurred in closed, open, and inactivated channels. Analysis of the voltage-dependence of block displayed a reduction of inhibition at positive membrane potentials. Frequency-dependence was not observed. Prazosin caused a negative shift in the voltage-dependence of both activation (-3.8 mV) and inactivation (-9.4 mV). The S6 mutations Y652A and F656A partially attenuated (Y652A) or abolished (F656A) HERG current blockade, indicating that prazosin binds to a common drug receptor within the pore-S6 region. In conclusion, this study demonstrates that HERG potassium channels are blocked by prazosin, doxazosin, and terazosin. These data may provide a hypothetical molecular explanation for the apoptotic effect of quinazoline-derived alpha1-adrenoceptor antagonists.[3] Metastatic prostate cancer progresses from androgen-dependent to androgen-independent. Terazosin, a long-acting selective alpha1-adrenoreceptor antagonist, induces apoptosis of prostate cancer cells in an alpha1-adrenoreceptor-independent manner, while genistein, a major soy isoflavone, inhibits the growth of several types of cancer cells. The present study was designed to test the therapeutic potential of a combination of terazosin and genistein using a metastatic, hormone-independent prostatic cancer cell line, DU-145. Terazosin or genistein treatment inhibited the growth of DU-145 cells in a dose-dependent manner, whereas had no effect on normal prostate epithelial cells. Addition of 1 microg/ml of terazosin, which was inactive alone, augmented the growth inhibitory effect of 5 microg/ml of genistein. Co-treatment with terazosin resulted in the genistein-induced arrest of DU-145 cells in G2/M phase being overridden and an increase in apoptotic cells, as evidenced by procaspase-3 activation and PARP cleavage. The combination also caused a greater decrease in the levels of the apoptosis-regulating protein, Bcl-XL, and of VEGF165 and VEGF121 than genistein alone. In conclusion, the terazosin/genistein combination was more effective in inhibiting cell growth and VEGF expression as well as inducing apoptosis of the metastatic, androgen-independent prostate cancer cell line, DU-145, than either alone. The doses used in this study are in lower and nontoxic anticancer dosage range, suggesting this combination has potential for therapeutic use.[4] |
| Molecular Formula |
C19H30CLN5O6
|
|
|---|---|---|
| Molecular Weight |
459.92
|
|
| Exact Mass |
459.188
|
|
| Elemental Analysis |
C, 49.62; H, 6.57; Cl, 7.71; N, 15.23; O, 20.87
|
|
| CAS # |
70024-40-7
|
|
| Related CAS # |
(R)-Terazosin; 109351-34-0; (S)-Terazosin; 109351-33-9; Terazosin; 63590-64-7; Terazosin hydrochloride; 63074-08-8
|
|
| PubChem CID |
63016
|
|
| Appearance |
White to off-white solid powder
|
|
| Boiling Point |
664.5ºC at 760 mmHg
|
|
| Melting Point |
215 - 217ºC
|
|
| LogP |
2.314
|
|
| Hydrogen Bond Donor Count |
4
|
|
| Hydrogen Bond Acceptor Count |
10
|
|
| Rotatable Bond Count |
4
|
|
| Heavy Atom Count |
31
|
|
| Complexity |
544
|
|
| Defined Atom Stereocenter Count |
0
|
|
| SMILES |
Cl[H].O1C([H])([H])C([H])([H])C([H])([H])C1([H])C(N1C([H])([H])C([H])([H])N(C2N=C(C3=C([H])C(=C(C([H])=C3N=2)OC([H])([H])[H])OC([H])([H])[H])N([H])[H])C([H])([H])C1([H])[H])=O.O([H])[H].O([H])[H]
|
|
| InChi Key |
NZMOFYDMGFQZLS-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C19H25N5O4.ClH.2H2O/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14;;;/h10-11,14H,3-9H2,1-2H3,(H2,20,21,22);1H;2*1H2
|
|
| Chemical Name |
[4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl]-(oxolan-2-yl)methanone;dihydrate;hydrochloride
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (5.44 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (5.44 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 2.5 mg/mL (5.44 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 4: 4.55 mg/mL (9.89 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication (<60°C). |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1743 mL | 10.8715 mL | 21.7429 mL | |
| 5 mM | 0.4349 mL | 2.1743 mL | 4.3486 mL | |
| 10 mM | 0.2174 mL | 1.0871 mL | 2.1743 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04551040 | Active Recruiting |
Drug: Terazosin | Healthy | University of Iowa | March 26, 2021 | Phase 1 |
| NCT04760860 | Not yet recruiting | Drug: Terazosin Hydrochloride Other: Placebo |
Dementia With Lewy Bodies | Qiang Zhang | October 2024 | Phase 1 Phase 2 |
| NCT04386317 | Recruiting | Drug: Terazosin | REM Sleep Behavior Disorder Pre-motor Parkinson's Disease |
Cedars-Sinai Medical Center | November 1, 2020 | Phase 2 |
| NCT05109364 | Recruiting | Drug: Terazosin therapy | REM Sleep Behavior Disorder Pre-motor Parkinson's Disease |
Cedars-Sinai Medical Center | September 23, 2022 | Phase 2 |
| NCT05855577 | Not yet recruiting | Drug: Terazosin | Parkinson Disease Gait Analysis Metabolic Disease |
I.R.C.C.S. Fondazione Santa Lucia |
December 2023 | Phase 4 |
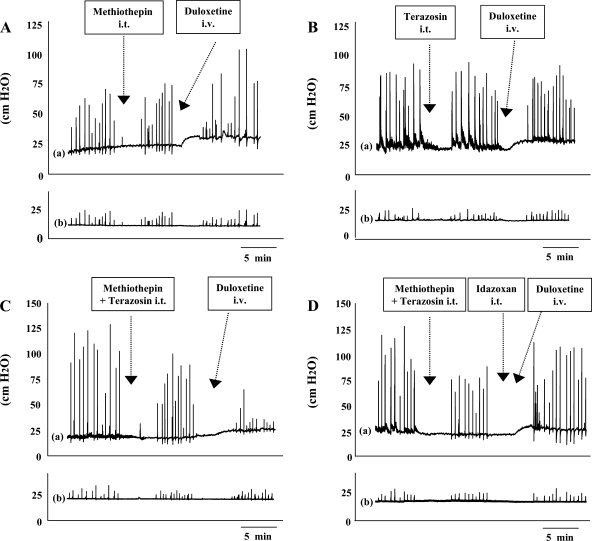 Representative traces of urethral (a) and abdominal (b) pressure changes induced by duloxetine (1 mg/kg iv) in the presence of intrathecal (it) methiothepin maleate (A), terazosin (B), coapplication of methiothepin maleate and terazosin (C), and coapplication.Am J Physiol Renal Physiol. 2008 Jul; 295(1): F264–F271. Representative traces of urethral (a) and abdominal (b) pressure changes induced by duloxetine (1 mg/kg iv) in the presence of intrathecal (it) methiothepin maleate (A), terazosin (B), coapplication of methiothepin maleate and terazosin (C), and coapplication.Am J Physiol Renal Physiol. 2008 Jul; 295(1): F264–F271. |
|---|
 |
  |