
| Size | Price | Stock | Qty |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g | |||
| Other Sizes |
Purity: ≥98%
Sunitinib (formerly also known as SU11248; trade name: Sutent) is a potent, orally bioavailable and multi-targeted RTK (receptor tyrosine kinase) inhibitor with potential antitumor activity. In cell-free assays, it inhibits c-Kit in addition to VEGFR2 (Flk-1) and PDGFRβ, with IC50s of 80 nM and 2 nM, respectively. In 2006, the US FDA approved sunitinib for the treatment of gastrointestinal stromal tumors that were resistant to imatinib and renal cell carcinoma. Sunitinib inhibits angiogenesis and cell proliferation by blocking the tyrosine kinase activities of platelet-derived growth factor receptor b (PDGFRb), c-kit, and vascular endothelial growth factor receptor 2 (VEGFR2).
| Targets |
PDGFRβ (IC50 = 2 nM); VEGFR2 (IC50 = 80 nM); FLT3; c-Kit
|
|---|---|
| ln Vitro |
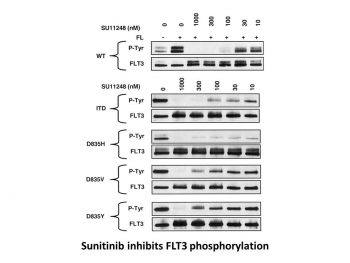
Sunitinib inhibits FLT-3 and Kit with considerable potency.[1] With a Ki of 9 nM and 8 nM, respectively, sunitinib is a strong ATP-competitive inhibitor of VEGFR2 (Flk1) and PDGFRβ. It provides >10-fold greater selectivity for VEGFR2 and PDGFR than FGFR-1, EGFR, Cdk2, Met, IGFR-1, Abl, and src. With IC50 values of 10 nM and 10 nM, respectively, sunitinib inhibits the phosphorylation of VEGFR2 in response to VEGF and PDGFRβ in response to PDGF in serum-starved NIH-3T3 cells expressing VEGFR2 or PDGFRβ. Sunitinib has an IC50 of 40 nM for VEGF-induced proliferation of serum-starved HUVECs and an IC50 of 39 nM and 69 nM for PDGF-induced proliferation of NIH-3T3 cells overexpressing PDGFRβ or PDGFRβ, respectively.[2] With an IC50 of 250 nM, 50 nM, and 30 nM, respectively, sunitinib inhibits the phosphorylation of wild-type FLT3, FLT3-ITD, and FLT3-Asp835. With IC50 values of 8 nM and 14 nM, respectively, sunitinib suppresses the growth of MV4;11 and OC1-AML5 cells and, in a dose-dependent fashion, triggers apoptosis.[3]
|
| ln Vivo |
Sunitinib (20–80 mg/kg/day) exhibits broad and potent dose-dependent anti-tumor activity against a variety of tumor xenograft models, including HT-29, A431, Colo205, H-460, SF763T, C6, A375, or MDA-MB-435. This is consistent with the significant and selective inhibition of VEGFR2 or PDGFR phosphorylation and signaling in vivo. Six out of eight mice receiving 80 mg/kg/day of sunitinib for 21 days experience complete tumor regression, and 110 days after the end of the treatment, there is no regrowth of the tumor.Tumors that do not completely regress after the first round of treatment can still be successfully treated with sunitinib in a second round. Tumor MVD significantly decreases with sunitinib treatment, with SF763T glioma tumors reduced by approximately 40%. Tumor size remains unchanged, but luciferase-expressing PC-3M xenografts treated with SU11248 completely inhibits further tumor growth.[2] Treatment with sunitinib (20 mg/kg/day) increases survival in the FLT3-ITD bone marrow engraftment model and significantly suppresses the growth subcutaneous MV4;11 (FLT3-ITD) xenografts.[3]
|
| Enzyme Assay |
Sunitinib's IC50 values against PDGFRβ and VEGFR2 (Flk-1) are ascertained by employing glutathione S-transferasefusion proteins that encompass the entire RTK cytoplasmic domain. In order to measure the trans-phosphorylation activity of VEGFR2 (Flk-1) and PDGFRβ, biochemical tyrosine kinase assays are carried out in 96-well microtiter plates that have been precoated (20 μg/well in PBS) and incubated with the peptide substrate poly-Glu,Tyr (4:1) for an entire night at 4 °C. Adding 1-5% (w/v) BSA to PBS blocks excess protein binding sites. The cells of insects infected with baculovirus produce purified GST-fusion proteins. The microtiter wells are then filled with GST-VEGFR2 and GST-PDGFRβ in a 2 × concentration kinase dilution buffer that contains 40 μM NaVO4, 50 mM NaCl, 100 mM HEPES, and 0.02% (w/v) BSA. 50 ng/mL is the final enzyme concentration for GST-VEGFR2 or GST-PDGFRβ. To create a range of inhibitor concentrations suitable for every enzyme, 25 μL of diluted Sunitinib is then added to each reaction well. A solution of MnCl2 is mixed with varying concentrations of ATP to start the kinase reaction. The final concentration of MnCl2 is 10 mM, and the final ATP concentrations span the Km for the enzyme. After allowing the plates to sit at room temperature for five to fifteen minutes, the reaction is halted by adding EDTA. After that, TBST is used to wash the plates three times. After adding rabbit polyclonal antiphosphotyrosine antisera at a 1:10,000 dilution to the wells in TBST containing 0.025% (w/v) nonfat dry milk, 0.5% (w/v) BSA, and 100 μM NaVO4, the wells are incubated at 37 °C for one hour. After three TBST washes, the plates are inoculated with goat anti-rabbit antisera conjugated with horseradish peroxidase (1:10,000 dilution in TBST). The plates are cleaned three times with TBST after an hour of incubation at 37 °C. Once 2,2′-azino-di-[3-ethylbenzthiazoline sulfonate] has been added as substrate, the amount of phosphotyrosine in each well is quantified.
|
| Cell Assay |
The cells are starved for an entire night in a medium containing 0.1% FBS before FL (50 ng/mL; FLT3-WT cells only) and sunitinib are added. After 48 hours of culture, proliferation is assessed using trypan blue cell viability assays or the Alamar Blue assay. Apoptosis is quantified using Western blotting 24 hours after Sunitinib addition in order to identify caspase-3 levels or poly (ADP-ribose) polymerase (PARP) cleavage.
|
| Animal Protocol |
Mice: The mice used are female nu/nu (8–12 weeks old, 25 grams). In short, on day 0, mice receive a subcutaneous injection of 3-5×106 tumor cells into the hind flank region. After tumors reach the indicated average size, mice bearing tumors are treated daily orally with carboxymethyl cellulose suspension or as a citrate buffered (pH 3.5) solution containing sunitinib. Tumor growth is assessed using tumor volume measurements taken twice a week. When tumors in animals receiving vehicle treatment reach an average size of 1000 mm3 or are determined to negatively impact the animals' quality of life, studies are usually stopped.
Rats: The Wistar rats are adult males weighing between 325 and 349 g. In two drug studies, the efficacy of the time-lapse imaging method in assessing the anti-angiogenic effects of a particular drug treatment is verified. First, mesenteric windows are taken from adult male Wistar rats, and the tissues are cultured for three days in two different experimental groups: 1) 10% serum (n = 8 tissues from 4 rats), and 2) 10% serum + Sunitinib (5 μM; n=8 tissues from 4 rats). |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
Maximum plasma concentrations (Cmax) of sunitinib are generally observed between 6 and 12 hours (Tmax) following oral administration. Food has no effect on the bioavailability of sunitinib. Sunitinib may be taken with or without food. The pharmacokinetics were similar in healthy volunteers and in the solid tumor patient populations tested, including patients with GIST and RCC. Sunitinib is metabolized primarily by the cytochrome P450 enzyme, CYP3A4, to produce its primary active metabolite, which is further metabolized by CYP3A4. Elimination is primarily via feces. In a human mass balance study of [14C]sunitinib, 61% of the dose was eliminated in feces, with renal elimination accounting for 16% of the administered dose. 2230 L (apparent volume of distribution, Vd/F) 34 - 62 L/h [Total oral clearance] Following oral administration, peak plasma concentrations of sunitinib generally occur within 6-12 hours. Food has no effect on bioavailability of sunitinib. Steady-state concentrations of sunitinib and its primary active metabolite are achieved within 10 to 14 days. By Day 14, combined plasma concentrations of sunitinib and its active metabolite ranged from 62.9 - 101 ng/mL. No significant changes in the pharmacokinetics of sunitinib or the primary active metabolite were observed with repeated daily administration or with repeated cycles in the dosing regimens tested. Sunitinib and its primary active metabolite are 95 and 90% bound to human plasma proteins in vitro, respectively. The apparent volume of distribution (Vd/F) for sunitinib was 2230 L. In the dosing range of 25 - 100 mg, the area under the plasma concentration-time curve (AUC) and Cmax increase proportionately with dose. For more Absorption, Distribution and Excretion (Complete) data for Sunitinib (11 total), please visit the HSDB record page. Metabolism / Metabolites Sunitinib is metabolized primarily by the cytochrome P450 enzyme, CYP3A4, to produce its primary active metabolite, which is further metabolized by CYP3A4. Sunitinib is metabolized principally by cytochrome P-450 (CYP) isoenzyme 3A4 to several metabolites. The main circulating metabolite, an N-desethyl derivative, has been shown to be equipotent to sunitinib in biochemical and cellular assays; this metabolite accounts for approximately 23-37% of total plasma concentrations of the drug and also is metabolized by CYP3A4. Sunitinib and its primary active metabolite were the major drug-related compounds identified in plasma, urine, and feces, representing 91.5%, 86.4% and 73.8% of radioactivity in pooled samples, respectively. Biological Half-Life Following administration of a single oral dose in healthy volunteers, the terminal half-lives of sunitinib and its primary active metabolite are approximately 40 to 60 hours and 80 to 110 hours, respectively. Following oral administration of a single dose in healthy volunteers, the terminal half-life of sunitinib or its primary active metabolite is approximately 40-60 or 80-110 hours, respectively. |
| Toxicity/Toxicokinetics |
Hepatotoxicity
In large clinical trials of sunitinib, elevations in serum aminotransferase levels were common, occurring in 39% of sunitinib vs 23% of placebo recipients. Values greater than 5 times the upper limit of normal (ULN) occurred in only 2% to 3% of sunitinib recipients (and 1% of controls). These abnormalities were usually asymptomatic. Dose adjustment or temporary discontinuation and restarting at a lower dose is recommended if enzyme levels are markedly elevated (ALT or AST persistently greater than 5 times ULN or bilirubin more than 3 times ULN). Sunitinib therapy is also associated with a high rate of serum bilirubin elevations, generally in the mild-to-moderate range and not in association with ALT or AST elevations. These changes are probably due to interaction with hepatic UDP-glucuronyltransferase, the enzyme that is also responsible for bilirubin excretion. More importantly, there have been several case reports of clinically apparent liver injury attributed to sunitinib therapy. The time to onset was after several cycles of therapy. The pattern of serum enzyme elevations was typically hepatocellular and the clinical presentation resembled acute hepatic necrosis. In some instances, the injury may have been due to hypotension, shock or ischemia rather than direct hepatotoxicity (Case 1). Regardless, the injury can be severe and several instances of acute liver failure and death have been reported. Immunoallergic features (rash, fever and eosinophilia) are not common. Finally, sunitinib has also been reported to cause hyperammonemia and encephalopathy in rare patients with cancer treated with conventional or even low oral doses (Case 2). The time to onset was within 1 to 3 weeks, presenting with confusion and irritability with minimal elevations in serum enzymes and bilirubin and marked increases (4-10 times the ULN) in serum ammonia. Recovery is rapid once sunitinib is stopped and the syndrome can recur with re-exposure. Interesting, there appears to be little cross-reactivity to this complication with other tyrosine kinase inhibitors. Likelihood score: B (highly likely cause of clinically apparent liver injury, including hyperammonemic syndrome). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of sunitinib during breastfeeding. Because sunitinib and its metabolite are over 90% bound to plasma proteins, the amount in milk is likely to be low. However, one of its metabolites has a half-life of up to 110 hours, and might accumulate in the infant. The manufacturer recommends that breastfeeding be discontinued during sunitinib therapy and for at least 4 weeks after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Binding of sunitinib and its primary metabolite to human plasma protein in vitro was 95% and 90%, respectively. Interactions ... A 57-year-old woman who started sunitinib treatment for relapsed metastatic gastrointestinal stromal tumor after imatinib failure had disease stabilization and normal liver function through 8 cycles of sunitinib 50 mg/day for 4 weeks, followed by 2 weeks off treatment. Her continuing medications included acetaminophen approximately 4.5 g/wk, as well as standard medications for asthma. In cycle 8, she received oral levothyroxine 50-150 microg/day for approximately 30 days to control hypothyroidism before beginning cycle 9 of sunitinib. On day 4 of cycle 9, she was hospitalized with progressively rising circulating liver enzyme levels. She died 4 days postadmission despite discontinuation of sunitinib and initiation of intensive supportive treatment. At autopsy, her liver showed severe centrilobular necrosis with moderate-to-severe steatosis and minimal parenchymal invasion by the neoplasm. Viral stains were negative. Hepatic failure has been reported rarely in patients receiving sunitinib. Autopsy results excluded neoplastic disease progression and viral infection in the etiology of the event, and the patient may have died of the combined interaction of sunitinib, acetaminophen, and levothyroxine. Although sunitinib was not more than a possible hepatotoxin (Roussel Uclaf Causality Assessment Method) and may even have been hepatoprotective over a 48-week period against chronic intake of acetaminophen (probable hepatotoxin) by producing regional hypothyroidism within the liver, it is hypothesized that correction of the putative hepatic hypothyroidism with oral levothyroxine (possible hepatotoxin) and reinitiation of sunitinib treatment may have triggered hepatic necrosis. ... Strong CYP3A4 inhibitors such as ketoconazole may increase sunitinib plasma concentrations. Selection of an alternate concomitant medication with no or minimal enzyme inhibition potential is recommended. Concurrent administration of SUTENT with the strong CYP3A4 inhibitor, ketoconazole, resulted in 49% and 51% increases in the combined (sunitinib + primary active metabolite) Cmax and AUC0-infinity values, respectively, after a single dose of Sunitinib in healthy volunteers. Co-administration of sunitinib with strong inhibitors of the CYP3A4 family (e.g., ketoconazole, itraconazole, clarithromycin, atazanavir, indinavir, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin, voriconazole) may increase sunitinib concentrations. Grapefruit may also increase plasma concentrations of sunitinib. CYP3A4 inducers such as rifampin may decrease sunitinib plasma concentrations. Selection of an alternate concomitant medication with no or minimal enzyme induction potential is recommended. Concurrent administration of sunitinib with the strong CYP3A4 inducer, rifampin, resulted in a 23% and 46% reduction in the combined (sunitinib + primary active metabolite) Cmax and AUC0-infinity values, respectively, after a single dose of sunitinib in healthy volunteers. Co-administration of sunitinib with inducers of the CYP3A4 family (e.g., dexamethasone, phenytoin, carbamazepine, rifampin, rifabutin, rifapentin, phenobarbital) may decrease sunitinib concentrations. St. John's wort may cause unpredictable decreases in plasma sunitinib concentrations and should be avoided during sunitinib therapy. |
| References | |
| Additional Infomation |
Therapeutic Uses
Angiogenesis Inhibitors; Antineoplastic Agents Sunitinib malate is indicated for the treatment of gastrointestinal stromal tumor after disease progression on or intolerance to imatinib mesylate. /Included in US product label/ Sunitinib malate is indicated for the treatment of advanced renal cell carcinoma. /Included in US product label/ Sunitinib malate is indicated for the treatment of progressive, well-differentiated pancreatic neuroendocrine tumors in patients with unresectable locally advanced or metastatic disease. /Included in US product label/ Drug Warnings /BOXED WARNING/ HEPATOTOXICITY-Hepatotoxicity has been observed in clinical trials and post-marketing experience. This hepatotoxicity may be severe, and deaths have been reported. Sunitinib has been associated with hepatotoxicity, which may result in liver failure or death. Liver failure has been observed in clinical trials (7/2281 [0.3%]) and post-marketing experience. Liver failure signs include jaundice, elevated transaminases and/or hyperbilirubinemia in conjunction with encephalopathy, coagulopathy, and/or renal failure. Monitor liver function tests (ALT, AST, bilirubin) before initiation of treatment, during each cycle of treatment, and as clinically indicated. Sunitinib should be interrupted for Grade 3 or 4 drug-related hepatic adverse events and discontinued if there is no resolution. Do not restart Sunitinib if patients subsequently experience severe changes in liver function tests or have other signs and symptoms of liver failure. Safety in patients with ALT or AST >2.5 x ULN or, if due to liver metastases, >5.0 x ULN has not been established. Cardiovascular events, including heart failure, myocardial disorders and cardiomyopathy, some of which were fatal, have been reported through post-marketing experience. Among patients receiving sunitinib for metastatic renal cell cancer in the randomized trial, 21% had a left ventricular ejection fraction (LVEF) below the lower limit of normal, and 4% experienced a decline in LVEF (to a value below 50% or as a reduction greater than 20% from the baseline value). Left ventricular dysfunction was reported in 1% and congestive heart failure in less than 1% of patients receiving sunitinib. For more Drug Warnings (Complete) data for Sunitinib (33 total), please visit the HSDB record page. Pharmacodynamics Sunitinib is an oral, small-molecule, multi-targeted receptor tyrosine kinase (RTK) inhibitor that was approved by the FDA on January 26, 2006. |
| Molecular Formula |
C22H27FN4O2
|
|---|---|
| Molecular Weight |
398.47
|
| Exact Mass |
398.211
|
| Elemental Analysis |
C, 66.31; H, 6.83; F, 4.77; N, 14.06; O, 8.03
|
| CAS # |
557795-19-4
|
| Related CAS # |
Sunitinib Malate;341031-54-7;Sunitinib-d10;1126721-82-1;Sunitinib-d4;1126721-79-6; 342641-94-5; 1275588-72-1 (mesylate) ; 1126641-10-8; 1327155-72-5 (HCl); 1221149-36-5 (acetate); 1332306-95-2 (oxalate)
|
| PubChem CID |
5329102
|
| Appearance |
Yellow solid powder
|
| Density |
1.2±0.1 g/cm3
|
| Boiling Point |
572.1±50.0 °C at 760 mmHg
|
| Melting Point |
189-191ºC
|
| Flash Point |
299.8±30.1 °C
|
| Vapour Pressure |
0.0±1.6 mmHg at 25°C
|
| Index of Refraction |
1.611
|
| LogP |
3.15
|
| Hydrogen Bond Donor Count |
3
|
| Hydrogen Bond Acceptor Count |
4
|
| Rotatable Bond Count |
7
|
| Heavy Atom Count |
29
|
| Complexity |
636
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
FC1C=C2C(NC(=O)/C/2=C\C2NC(C)=C(C(NCCN(CC)CC)=O)C=2C)=CC=1
|
| InChi Key |
WINHZLLDWRZWRT-ATVHPVEESA-N
|
| InChi Code |
InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12-
|
| Chemical Name |
N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-2-oxo-1H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide
|
| Synonyms |
SU11248; SU 11248; sunitinibum; Su-011248; Sunitinib Base; SU011248; SU-11248; sunitinib; trade name: Sutent.
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: This product requires protection from light (avoid light exposure) during transportation and storage. |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 1.11 mg/mL (2.79 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 11.1 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 1.11 mg/mL (2.79 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 11.1 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. View More
Solubility in Formulation 3: 5% DMSO+corn oil: 7mg/mL |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5096 mL | 12.5480 mL | 25.0960 mL | |
| 5 mM | 0.5019 mL | 2.5096 mL | 5.0192 mL | |
| 10 mM | 0.2510 mL | 1.2548 mL | 2.5096 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
TAPUR: Testing the Use of Food and Drug Administration (FDA) Approved Drugs That Target a Specific Abnormality in a Tumor Gene in People With Advanced Stage Cancer
CTID: NCT02693535
Phase: Phase 2 Status: Recruiting
Date: 2024-11-12
|
 |