
| Size | Price | Stock | Qty |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| Other Sizes |
|
Purity: = 99.52%
Sumatriptan Succinate (GR-43175; GR43175; Sumatran; Sumax), an approved triptan sulfa drug and a sulfonamide compound, is a selective 5-HT1 receptor agonist used for the treatment of migraine headaches. It is selective to 5-HT1A, 5-HT1B, and 5-HT1D. Its succinate salt is called sumatriptan succinate.
| Targets |
5-HT1D Receptor ( Ki = 17 nM ); 5-HT1B Receptor ( Ki = 27 nM ); 5-HT1A Receptor ( IC50 = 100 nM )
|
|---|---|
| ln Vitro |
In vitro activity: Sumatriptan is slightly less effective at 5-HT1A binding sites (Ki = 100 nM) but exhibits the highest affinity for 5-HT1D (Ki = 17 nM) and 5-HT1B (Ki = 27 nM) binding sites. The effects of electrical stimulation of the trigeminal ganglion on plasma protein extravasation are significantly reduced by sumatriptan. The morphological alterations in mast cells and post-capillary venules within the dura mater that occur after electrical trigeminal ganglion stimulation are lessened by sumatriptan.
Aims: Sumatriptan is a 5-HT1B/1D-receptor agonist which also has affinity for 5-HT1F-receptors. The vasoconstrictor effects of sumatriptan are thought to be 5-HT1B-receptor mediated and these receptors have been shown to be expressed in human cranial blood vessels. However, in the same tissue mRNA coding for 5-HT1F-receptors has also been identified and this study addresses the possibility of whether 5-HT1F-receptor activation contributes to vasoconstriction. Methods: The ability of two selective 5-HT1B/1D-receptor antagonists (GR125,743 and GR127,935) with no affinity for 5-HT1F-receptors, to inhibit sumatriptan evoked contractions in human isolated middle meningeal artery was investigated. Using a series of 5-HT1B/1D-receptor agonists (sumatriptan, zolmitriptan, CP122,288, L-741,519 and L-741,604), some with high affinity for 5-HTIF-receptors and the non-selective 5-HT-receptor agonists 5-HT and 5-CT, we compared the vasoconstrictor potency of these drugs in human isolated middle meningeal artery with their affinities at cloned human 5-HT1B-, 5-HT1D-and 5-HT1F-receptors expressed in CHO cell lines. Results: GR125,743 antagonized sumatriptan evoked contractions in a competitive manner (apparent pA2 9.1) and GR127,935 antagonized sumatriptan-induced responses in a non-competitive manner (reducing the maximum contraction to 27%). There was a significant correlation between vasoconstrictor potency and 5-HT1B-receptor affinity (r=0.93, P=0.002) but not with 5-HT1D- or 5-HT1F-receptor affinity (r=0.74, P=0.06; r= 0.31, P= 0.49, respectively). Conclusions: These experiments show that in human middle meningeal artery vasoconstriction to sumatriptan-like agents is 5-HT1B-receptor mediated with little if any contribution from 5-HT1F-receptor activation[3]. |
| ln Vivo |
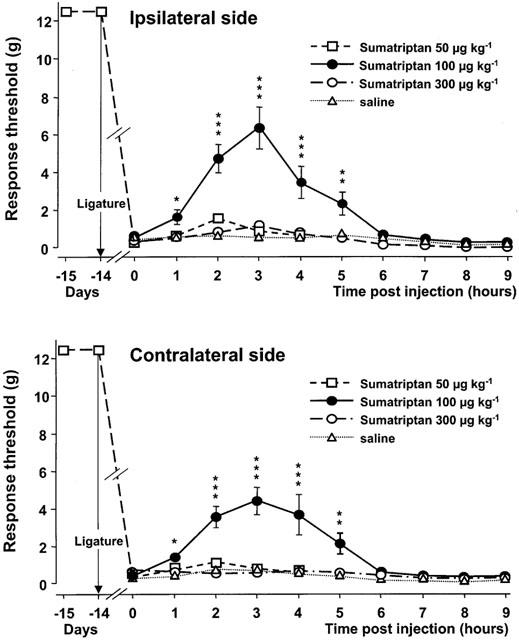
In rats with a trigeminal neuropathic pain model, sumatriptan at a clinically relevant dose (100 mg/kg, s.c.) significantly reduces the mechanical allodynia-like behavior on both the injured and contralateral sides (peak-effects 6.3 g and 4.4 g, respectively). Following mechanical stimulation in cats, sumatriptan decreases the number of Fos-positive cells (6, 13 cells and 9 cells, respectively) in laminae I and IIo of the trigeminal nucleus caudalis and C2. Sumatriptan selectively constricts the cranial vessels that are enlarged and inflamed during a migraine attack; this action is mediated by activating a subtype of 5-HT1 receptor that has been localized in cranial vessels in animals. Oral bioavailabilities of sumatriptan in rats, dogs, and rabbits are 37, 58, and 23%, respectively. In rats, dogs, and rabbits, sumatriptan has a half-life of 1-2 hours and is rapidly eliminated by metabolic and renal processes. Although it is less well tolerated in dogs, sumatriptan has few negative pharmacodynamic effects when given acutely, with the exception of high doses.
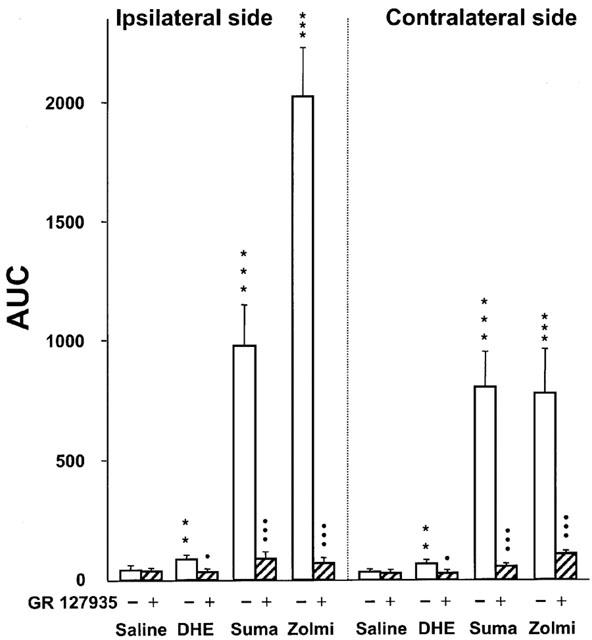
Sumatriptan is a serotonin1 (5-HT1) receptor agonist, which is effective in the acute treatment of migraine headache. Its antimigraine activity is believed to derive from selective vasoconstriction of cranial blood vessels which are dilated and distended during migraine headache and/or from inhibition of neurogenically mediated inflammation in the dura mater. In placebo-controlled comparative studies, sumatriptan reduced migraine headache from 'moderate or severe' to 'mild or none' within 2 hours in 50 to 73% of patients following oral administration of 100 or 200 mg, and within 1 hour in 70 to 80% of patients following subcutaneous doses of 6 to 8 mg or intranasal doses 20 mg into each nostril. In addition, sumatriptan alleviated the accompanying symptoms of nausea, vomiting, and photophobia/phonophobia more effectively than placebo, and permitted higher percentages of patients to resume normal daily activities. Sumatriptan 100 mg orally was more effective in the acute treatment of migraine than oral combination therapy consisting of ergotamine 2 mg plus caffeine 200 mg or aspirin 900 mg plus metoclopramide 10 mg. Pooled data from nearly 5000 patients treated with either oral or subcutaneous sumatriptan in clinical trials indicate that it is well tolerated. However, migraine recurrence within 24 or 48 hours of initial symptom resolution developed in approximately 40% of patients treated with sumatriptan, irrespective of route of administration. It is likely that migraine recurrence is related to the short half-life of the drug (approximately 2 hours). Future studies should attempt to ascertain whether additional doses of sumatriptan will help prevent migraine recurrence in patients with attacks of long duration and if so, should determine the optimum interval between dosages. In conclusion, sumatriptan is an important addition to the range of drugs currently available for acute treatment of migraine. It provides rapid relief from debilitating symptoms in a high percentage of patients, particularly after subcutaneous administration. At this stage in its development a number of questions remain to be answered - most notably whether repeat doses will help prevent recurrent attacks and which patients are most likely to respond to therapy. Nevertheless, sumatriptan presently offers a combination of efficacy and tolerability that is unique in this particular clinical setting[2]. The association between the clinical use of nitroglycerin (NTG) and headache has led to the examination of NTG as a model trigger for migraine and related headache disorders, both in humans and laboratory animals. In this study in mice, we hypothesized that NTG could trigger behavioural and physiological responses that resemble a common manifestation of migraine in humans. We report that animals exhibit a dose-dependent and prolonged NTG-induced thermal and mechanical allodynia, starting 30-60 min after intraperitoneal injection of NTG at 5-10 mg/kg. NTG administration also induced Fos expression, an anatomical marker of neuronal activity in neurons of the trigeminal nucleus caudalis and cervical spinal cord dorsal horn, suggesting that enhanced nociceptive processing within the spinal cord contributes to the increased nociceptive behaviour. Moreover, sumatriptan, a drug with relative specificity for migraine, alleviated the NTG-induced allodynia. We also tested whether NTG reduces the threshold for cortical spreading depression (CSD), an event considered to be the physiological substrate of the migraine aura. We found that the threshold of CSD was unaffected by NTG, suggesting that NTG stimulates migraine mechanisms that are independent of the regulation of cortical excitability[4]. 1. Peripheral lesion to the trigeminal nerve may induce severe pain states. Several lines of evidence have suggested that the antimigraine effect of the triptans with 5-HT(1B/1D) receptor agonist properties may result from inhibition of nociceptive transmission in the spinal nucleus of the trigeminal nerve by these drugs. On this basis, we have assessed the potential antinociceptive effects of sumatriptan and zolmitriptan, compared to dihydroergotamine (DHE), in a rat model of trigeminal neuropathic pain. 2. Chronic constriction injury was produced by two loose ligatures of the infraorbital nerve on the right side. Responsiveness to von Frey filament stimulation of the vibrissal pad was used to evaluate allodynia. 3. Two weeks after ligatures, rats with a chronic constriction of the right infraorbital nerve displayed bilateral mechanical hyper-responsiveness to von Frey filament stimulation of the vibrissal pad with a mean threshold of 0.38+/-0.04 g on the injured side and of 0.43+/-0.04 g on the contralateral (left) side (versus > or =12.5 g on both sides in the same rats prior to nerve constriction injury). 4. Sumatriptan at a clinically relevant dose (100 microg kg(-1), s.c.) led to a significant reduction of the mechanical allodynia-like behaviour on both the injured and the contralateral sides (peak-effects 6.3+/-1.1 g and 4.4+/-0.7 g, respectively). A more pronounced effect was obtained with zolmitriptan (100 microg kg(-1), s.c.) (peak-effects: 7.4+/-0.9 g and 3.2+/-1.3 g) whereas DHE (50-100 microg kg(-1), i.v.) was less active (peak-effect approximately 1.5 g). 5. Subcutaneous pretreatment with the 5-HT(1B/1D) receptor antagonist, GR 127935 (3 mg kg(-1)), prevented the anti-allodynia-like effects of triptans and DHE. Pretreatment with the 5-HT(1A) receptor antagonist, WAY 100635 (2 mg kg(-1), s.c.), did not alter the effect of triptans but significantly enhanced that of DHE (peak effect 4.3+/-0.5 g). 6. In a rat model of peripheral neuropathic pain, which consisted of a unilateral loose constriction of the sciatic nerve, neither sumatriptan (50-300 microg kg(-1)) nor zolmitriptan (50-300 microg kg(-1)) modified the thresholds for paw withdrawal and vocalization in response to noxious mechanical stimulation. 7. These results support the rationale for exploring the clinical efficacy of brain penetrant 5-HT(1B/1D) receptor agonists as analgesics to reduce certain types of trigeminal neuropathic pain in humans[5]. |
| Enzyme Assay |
Binding studies: cell lines [3]
[3H]-5-HT displacement studies were carried out on membranes (approximately 6 mg wet weight per tube) prepared from CHO (chinese hamster ovary) cell lines expressing human 5-HT1B-, 5-HT1D- and 5-HT1F-receptors. 5-HT (10 μm) was used to define non-specific binding. Membranes, radioligand (2 nm) and competing drug were made up in 50 mm Tris HCl buffer (containing 10 μm pargyline, 0.1% ascorbate, 4 mm CaCl2, pH 7.7) and were incubated for 30 min at 37° C. The reaction was terminated by filtration through GF/B filters using a Brandel cell harvester. The ability of sumatriptan (GR 43175; 3-[2-dimethylamino]ethyl-N-methyl-1H-indole-5 methane sulphonamide) to interact with 13 neurotransmitter receptor sites was determined using radioligand binding techniques. Sumatriptan displayed the highest affinity for 5-HT1D (Ki = 17 nM) and 5-HT1B (Ki = 27 nM) binding sites and was slightly less potent at 5-HT1A binding sites (Ki = 100 nM). By contrast, sumatriptan was essentially inactive (Ki greater than 10,000 nM) at each of the 10 other binding sites analyzed. These data indicate that sumatriptan interacts selectively with 5-HT1B and 5-HT1D sites and suggest that these interactions may be the basis of its apparent efficacy in the acute treatment of migraine [1]. |
| Cell Assay |
Functional studies: human middle meningeal arteries [3]
Discarded pieces of dura mater containing segments of middle meningeal arteries were obtained from patients undergoing neurosurgery. The vessels were transported in modified physiological salt solution (4° C) to the laboratory where the arteries were dissected free from the dura mater. Ring segments (2–3 mm in length) were prepared and mounted for isometric tension recording in organ baths containing a standard physiological salt solution aerated with 95%CO2/5% O2, maintained at 37° C, pH 7.4. A resting tension of 4 g was applied. Most patients were prescribed Ca2+-channel antagonists pre-operatively and to remove any residual traces of medication, the segments were washed overnight with physiological salt solution at room temperature. The following day, the temperature was restored to 37° C (1 h equilibration) and the contractile response to the reference agonist KCl (45 mm) was determined. Once the maximum response to KCl was achieved a wash-off period followed (30 min). Cumulative concentration-effect curves to 5-HT and 5-CT and the selective 5-HT1B/1D-receptor agonists zolmitriptan, CP122,288, L-741,519 and L-741,604, were performed (1 nm-30 μm) where increasing concentrations of each drug were added once the previous challenge produced a plateau (2–3 min). Sumatriptan concentration-effect curves (10 nm–100 μm) were also carried out in the absence and presence of the antagonists GR125,743 or GR127,935 (10 nm, equilibration 30 min) where two consequetive concentration-effect curves were performed on a single segment. Vehicle controls were also performed in the same manner. Functional studies: cell lines [3] Membranes from the CHO cells expressing human 5-HT1B-and 5-HT1D-receptors were prepared essentially as described by Lazareno & Birdsall. The final pellets were resuspended in HEPES buffer (20 mm HEPES, 100 mm NaCl, 10 mm MgCl2, 0.1% ascorbate and 10 μm pargyline). Membranes (2.5 mg wet weight per tube) were incubated with 100 μm and 30 μm GDP for 5-HT1B- and 5-HT1D-receptors, respectively, and test drug for 20 min at 30° C, before being transferred to ice for 15 min. [35S]-GTPγS (100 pm) was added to all tubes and the tubes were incubated for a further 30 min at 30° C before being rapidly filtered over GF/B filters using a Brandel cell harvester. |
| Animal Protocol |
Sumatriptan administration [4]
Dilutions of Sumatriptan were made from an injectable preparation of sumatriptan succinate at 12 mg/ml. Five minutes after the administration of 10 mg/kg NTG, each animal was treated with i.p. sumatriptan (300 μg/kg, 600 μg/kg) or saline. Alternatively, an identical set of NTG-injected mice was treated with a single injection of intrathecal sumatriptan (0.06 μg in 5 μl) or saline at 5 min after NTG administration. Intrathecal (i.t.) injections were made in lightly restrained, awake mice using a 25-μl Hamilton syringe mounted onto a 30-G, 0.5-in needle inserted between the L4-5 lumbar interspace. As the effective i.t. dose of 0.06 μg is approximately 1/100 of the 300-μg/kg systemic dose, we believe that any observed effect is almost certainly via an action at a central target. Neither i.t. nor systemic sumatriptan at these doses produces any changes in acute nociceptive thresholds, nor do they cause any sensorimotor impairment that would interfere with these behavioural experiments |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
A 6mg subcutaneous injection of sumatriptan reaches a Cmax of 69.5ng/mL (95% CI of 62.8-76.9ng/mL) with a Tmax of 0.17h (95% CI of 0.08-0.33h), an AUC of 9.0h\*ng/mL (95% CI of 7.5-10.9h\*ng/mL), and a bioavailability of 100%. A 25mg oral dose of sumatriptan reaches a Cmax of 16.5ng/mL (95% CI of 13.5-20.1ng/mL) with a Tmax of 1.50h (95% CI of 0.50-2.00h), an AUC of 8.7h\*ng/mL (95% CI of 6.1-12.5h\*ng/mL), and a bioavailability of 14.3% (95% CI of 11.4-17.9%). A 20mg intranasal dose of sumatriptan reaches a Cmax of 12.9ng/mL (95% CI of 10.5-15.9ng/mL) with a Tmax of 1.50h (95% CI of 0.25-3.00h), an AUC of 7.4h\*ng/mL (95% CI of 5.0-10.8h\*ng/mL), and a bioavailability of 15.8% (95% CI of 12.6-19.8%). A 25mg rectal dose of sumatriptan reaches a Cmax of 22.9ng/mL (95% CI of 18.4-28.6ng/mL) with a Tmax of 1.00h (95% CI of 0.75-3.00h), an AUC of 14.6h\*ng/mL (95% CI of 11.3-18.8h\*ng/mL), and a bioavailability of 19.2% (95% CI of 15.3-24.1%). 22±4% is excreted in the urine as unchanged sumatriptan and 38±7% in urine as indole acetic acid approximately 40% is excreted in the feces. Sumatriptan has a volume of distribution of 50±8L for a 6mg subcutaneous dose, or 2.7L/kg. Subcutaneous sumatriptan has a clearance of 0.22L/min (95% CI of 0.19-0.25L/min). Oral sumatriptan has a clearance of 0.17L/min (95% CI of 0.14-0.21L/min). Rectal sumatriptan has a clearance of 0.17L/min (95% CI of 0.14-0.21L/min). Intrsnasal sumatriptan has a clearance of 0.21L/min (95% CI of 0.18-0.25L/min). Total plasma clearance of sumatriptan is approximately 1200mL/min. Sumatriptan is rapidly absorbed following subcutaneous or oral administration; oral absorption appears to occur in the small intestine. The drug also is absorbed rapidly following intranasal administration. The bioavailability of sumatriptan given subcutaneously is almost complete, averaging about 97% of that obtained with iv administration of the drug. The bioavailability of sumatriptan following oral or intranasal administration averages only about 15 or 17%, respectively, principally because of presystemic metabolism of the drug and in part because of incomplete absorption. The area under the plasma concentration-time curve (AUC) and peak serum concentration of sumatriptan increase linearly with single subcutaneous doses of 1-16 mg. The extent of sumatriptan absorption (AUC) also is dose-proportional following single oral doses of 25-200 mg; however, peak plasma concentrations after a 100-mg oral dose of sumatriptan are approximately 25% less than those predicted from a 25-mg oral dose. Interindividual variability in the absorption of sumatriptan after oral administration results in multiple peaks in plasma concentration, possibly because of differences in the rates of gastric emptying, small-bowel transit, and/or presystemic metabolism; however, 75-80% of the final peak plasma concentration is reached within 45 minutes after dosing. Administration of higher than recommended single oral doses of sumatriptan (ie, 200-400 mg) is associated with a decrease in the rate of absorption. Oral absorption of the drug does not appear to be affected appreciably by gastric stasis that may occur during a migraine attack; however, the time to peak concentration is prolonged by about 30 minutes. The pharmacokinetics of sumatriptan following subcutaneous injection reportedly are similar during migraine attacks and pain-free periods. Absorption of subcutaneous sumatriptan is not affected by race or gender. A food effect study involving administration of sumatriptan tablets to healthy volunteers under fasting conditions and with a high-fat meal indicated that the Cmax and AUC were increased by 15% and 12%, respectively, when administered in the fed state. For more Absorption, Distribution and Excretion (Complete) data for Sumatriptan (17 total), please visit the HSDB record page. Metabolism / Metabolites Sumatriptan is predominantly metabolized by monoamine oxidase A. The main metabolites are the inactive indole acetic acid and indole acetic acid glucuronide. The principal metabolite of sumatriptan is its inactive indole acetic acid analog, which is formed by oxidative N-deamination of the N-dimethyl side chain. The indole acetic acid metabolite of sumatriptan achieves plasma concentrations 6-7 times higher than those of sumatriptan but has a half-life similar to that of the parent compound, suggesting that clearance of this metabolite is formation-rate limited. Other minor metabolites of sumatriptan, an ester glucuronide of the indole acetic acid derivative and an indole ethyl alcohol derivative, also have been identified. Metabolism is the principal clearance process for sumatriptan. Sumatriptan is metabolized in the liver and possibly in the GI tract and is eliminated in urine and feces. In vitro studies suggest that sumatriptan is metabolized by monoamine oxidase (MAO), principally the A isoenzyme (MAO-A); inhibitors of this enzyme may increase systemic exposure to sumatriptan. Hepatic. In vitro studies with human microsomes suggest that sumatriptan is metabolized by monoamine oxidase (MAO), predominantly the A isoenzyme. Route of Elimination: Only 3% of the dose is excreted in the urine as unchanged sumatriptan; 42% of the dose is excreted as the major metabolite, the indole acetic acid analogue of sumatriptan. Half Life: 2.5 hours Biological Half-Life Subcutaneous sumatriptan has a half life of 1.9h (95% CI of 1.7-2.0h). Oral sumatriptan has a half life of 1.7h (95% CI of 1.4-1.9h). Rectal sumatriptan has a half life of 1.8h (95% CI of 1.6-2.2h). Intrsnasal sumatriptan has a half life of 1.8h (95% CI of 1.7-2.0h). Following single subcutaneous or oral doses of sumatriptan in healthy individuals, the terminal elimination half-life of the drug is 1.5-2.6 hours. Following single-dose oral administration of large doses of sumatriptan or repeated administration of smaller doses, a second terminal elimination phase has been observed but not characterized. The prolonged elimination half-life with multiple dosing or administration of large single doses may indicate enterohepatic recycling or prolonged oral absorption and does not appear to affect substantially the disposition of the drug. Most of a dose of sumatriptan is excreted within 10-24 hours. Following intranasal administration of sumatriptan, the elimination half-life reportedly is about 2 hours. |
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Because of the low levels of sumatriptan in breastmilk, amounts ingested by the infant are small. It also has poor oral bioavailability, further decreasing infant exposure to the drug. Some authors have suggested that withholding breastfeeding for 8 hours after a single subcutaneous injection would virtually eliminate infant exposure to the drug. The manufacturer recommends withholding breastfeeding for 12 hours after a dose. Withholding breastfeeding might be helpful in extreme cases, such as in the mother of a preterm infant, but sumatriptan would not be expected to cause any adverse effects in most breastfed infants. Painful, burning nipples and breast pain have been reported after doses of sumatriptan and other triptans. This has occasionally been accompanied by a decrease in milk production. ◉ Effects in Breastfed Infants One author reported correspondence with the drug's manufacturer stating that of 3 women known to the manufacturer who used sumatriptan (dose and route unspecified) during breastfeeding none reported adverse effects on the infants. ◉ Effects on Lactation and Breastmilk One author reported correspondence with the drug's manufacturer stating that 1 woman who used a single injection of sumatriptan (dose unspecified) during breastfeeding had a cessation of lactation. A review of four European adverse reaction databases found 26 reported cases of, painful, burning nipples, painful breasts, breast engorgement and/or painful milk ejection in women who took a triptan while nursing. Pain was sometimes intense and occasionally led to decreased milk production. Pain generally subsided with time as the drug was eliminated. The authors proposed that triptans may cause vasoconstriction of the arteries in the breast, nipples, and the arteries surrounding the alveoli and milk ducts, causing a painful sensation and a painful milk ejection reflex. ◈ What is sumatriptan? Sumatriptan is a medication that has been used to treat migraine headaches. It can be taken by mouth (orally) in pill form, by nasal spray, or by injection (shot). Some brand names for sumatriptan are Imitrex®, Alsuma®, Imigran®, Onzetra Xsail®, Tosymra®, and Zembrace SymTouch®.Sumatriptan is available in a combination product (Treximet®) that also contains naproxen. As of October 2020, the U.S. Food and Drug Administration (FDA) states people who are pregnant should not use NSAIDs (such as naproxen) after week 20 of pregnancy unless specifically advised to do so by their healthcare provider. For more information on naproxen, see our fact sheet at https://mothertobaby.org/fact-sheets/naproxen/.Sometimes when people find out they are pregnant, they think about changing how they take their medication, or stopping their medication altogether. However, it is important to talk with your healthcare providers before making any changes to how you take your medication. Your healthcare providers can talk with you about the benefits of treating your condition and the risks of untreated illness during pregnancy. ◈ Can having migraine headaches affect pregnancy? Some studies have shown that people with a history of migraine headaches have a slightly higher chance for pregnancy complications including high blood pressure, preeclampsia (high blood pressure and problems with organs, such as the kidneys), that can lead to seizures (called eclampsia), and pregnancy-related stroke. Be sure to talk with your healthcare providers about your history of migraine headaches so they can monitor symptoms during pregnancy, if needed. ◈ I take sumatriptan. Can it make it harder for me to get pregnant? It is not known if sumatriptan can make it harder to get pregnant. ◈ Does taking sumatriptan increase the chance of miscarriage? Miscarriage is common and can occur in any pregnancy for many different reasons. There have been several studies done that have not found an increased chance of miscarriage when sumatriptan was used during pregnancy. ◈ Does taking sumatriptan increase the chance of birth defects? Every pregnancy starts out with a 3-5% chance of having a birth defect. This is called the background risk. Overall, studies have not found an increased chance of birth defects when sumatriptan is used in the first trimester. ◈ Does taking sumatriptan in pregnancy increase the chance of other pregnancy-related problems? Some studies have suggested a small increase in chance of some pregnancy-related problems, including preeclampsia, preterm birth (birth before 37 weeks), low birth weight (weighing less than 5 pounds, 8 ounces [2500g] at birth), and heavy bleeding after delivery if sumatriptan was used late in pregnancy. However, some of these complications (including preeclampsia, preterm birth, and low birth weight) have been associated with migraines in pregnancy. This makes it hard to know if the medication, the condition being treated, or other factors are the cause of these complications. ◈ Does taking sumatriptan in pregnancy affect future behavior or learning for the child? Studies have not been done to see if sumatriptan can cause behavior or learning issues for the child. There is limited information on the long-term effects of exposure to medications in the same class as sumatriptan during pregnancy. One study that followed children to age 3 suggested there could be small effects on attention. However, the same authors did not find behavior differences at age 5 in children who were exposed to a medication in the same class as sumatriptan. ◈ Breastfeeding while taking sumatriptan: Sumatriptan gets into breast milk in small amounts and is not well absorbed by the stomach. The manufacturer states avoiding breastfeeding for 12 hours after a dose of sumatriptan can minimize infant exposure. While this may be helpful in some cases (such as when the breastfeeding infant was born preterm), sumatriptan is not expected to cause side effects in most breastfed infants. Be sure to talk to your healthcare provider about all your breastfeeding questions. ◈ If a male takes sumatriptan, could it affect fertility or increase the chance of birth defects? Studies have not been done to see if sumatriptan could affect male fertility (ability to get partner pregnant) or increase the chance of birth defects above the background risk. In general, exposures that fathers or sperm donors have are unlikely to increase risks to a pregnancy. For more information, please see the MotherToBaby fact sheet Paternal Exposures at https://mothertobaby.org/fact-sheets/paternal-exposures-pregnancy/. Protein Binding Sumatriptan is 14%-21% bound to protein in circulation. |
| References | |
| Additional Infomation |
Sumatriptan succinate is a succinate salt obtained by reaction of sumatriptan with one equivalent of succinic acid. Selective agonist for a vascular 5-HT1 receptor subtype (probably a member of the 5-HT1D family). Used for the acute treatment of migraine with or without aura in adults. It has a role as a serotonergic agonist and a vasoconstrictor agent. It contains a sumatriptan(1+).
Sumatriptan Succinate is the succinate salt form of sumatriptan, a member of the triptan class of compounds with anti-migraine property. Sumatriptan succinate selectively binds to and activates serotonin 5-HT1 receptors. This results in constriction of meningeal, dural, cerebral or pial blood vessels via stimulation of the 5-HT1B receptors, thereby reducing the vascular pulsation and may provide relief in migraine headaches. Furthermore, agonistic action of this agent through presynaptic stimulation of 5-HT1D and/or 5-HT1F receptors prevents release of vasoactive and pro-inflammatory neuropeptide (calcitonin gene-related peptide), thereby may also relieve migraine headaches. In addition, central inhibition of pain transmission via the inhibition of trigeminal neurons in the brain stems and upper spinal cord mediated by 5-HT1B, 5-HT1D or 5-HT 1F receptors also aides in the alleviation of migraine pain. A serotonin agonist that acts selectively at 5HT1 receptors. It is used in the treatment of MIGRAINE DISORDERS. See also: Sumatriptan (has active moiety); Naproxen Sodium; Sumatriptan Succinate (component of). Sumatriptan is a sulfonamide that consists of N,N-dimethyltryptamine bearing an additional (N-methylsulfamoyl)methyl substituent at position 5. Selective agonist for a vascular 5-HT1 receptor subtype (probably a member of the 5-HT1D family). Used (in the form of its succinate salt) for the acute treatment of migraine with or without aura in adults. It has a role as a serotonergic agonist and a vasoconstrictor agent. It is a sulfonamide and a member of tryptamines. It is functionally related to a N,N-dimethyltryptamine. It is a conjugate acid of a sumatriptan(1+). Sumatriptan is a serotonin receptor agonist commonly used to treat migraines and sometimes cluster headaches. Sumatriptan is the first of the triptans and was made available in Europe in 1991 to treat migraines. Sumatriptan was granted FDA approval on 28 December 1992. Sumatriptan is a Serotonin-1b and Serotonin-1d Receptor Agonist. The mechanism of action of sumatriptan is as a Serotonin 1b Receptor Agonist, and Serotonin 1d Receptor Agonist. Sumatriptan is a sulfonamide triptan with vasoconstrictor activity. Sumatriptan selectively binds to and activates serotonin 5-HT1D receptors in the central nervous system (CNS), thereby constricting cerebral blood vessels. This may lead to a relief in pain from vascular headaches. Sumatriptan may also relieve vascular headaches by decreasing the release of vasoactive neuropeptides from perivascular trigeminal axons in the dura mater during a migraine, by reducing extravasation of plasma proteins, and by decreasing the release of other mediators of inflammation from the trigeminal nerve. Oftentimes, serotonin levels in the brain become extremely erratic before the onset of a migraine. In an attempt to stabilize this, sumatriptan is administered to help aid in leveling the serotonin levels in the brain. Sumatriptan is structurally similar to serotonin, and is a 5-HT (5-HT1D) agonist, which is one of the receptors that serotonin binds to. The specific receptor subtype it activates is present in the cranial and basilar arteries. Activation of these receptors causes vasoconstriction of those dilated arteries. Sumatriptan is also shown to decrease the activity of the trigeminal nerve. Sumatriptan is a triptan drug including a sulfonamide group structurally similar to serotonin, and is a 5-HT (5-HT1D) agonist, which is one of the receptors that serotonin binds to. Oftentimes, serotonin levels in the brain become extremely erratic before the onset of a migraine. In an attempt to stabilize this, sumatriptan is administered to help aid in leveling the serotonin levels in the brain. A serotonin agonist that acts selectively at 5HT1 receptors. It is used in the treatment of migraines. Sumatriptan (Imitrex, Imigran, Imigran Recovery) is a triptan drug including a sulfonamide group which was originally developed by Glaxo for the treatment of migraine headaches. A serotonin agonist that acts selectively at 5HT1 receptors. It is used in the treatment of MIGRAINE DISORDERS. See also: Sumatriptan Succinate (has salt form); Sumatriptan; naproxen sodium (component of). Drug Indication A combination sumatriptan and [naproxen] tablet is indicated for the treatment of migraines with or without auras in patients 12 years of age and older. Sumatriptan nasal powder, nasal spray, subcutaneous injection, and tablets are indicated to treat migraines with or without auras in adults. One of the subcutaneous formulations of sumatriptan is also indicated to treat cluster headaches in adults, while the other subcutaneous formulation is not. Mechanism of Action Sumatriptan is an agonist of 5-HT1B and 5-HT1D. This agonism leads to constriction of cranial blood vessels and inhibits the release of pro-inflammatory neuropeptides. Sumatriptan decreases carotid arterial blood flow, but increases blood flow velocity in the internal carotid artery and middle cerebral artery.[A179734 Agonism of the 5-HT1B and 5-HT1D receptors also inhibits sensory neurons, preventing the release of vasoactive peptides.[A179734 Sumatriptan does not cross the blood brain barrier. Sumatriptan and other currently available drugs that are effective for acute migraine, including dihydroergotamine and ergotamine, have binding affinity for serotonin type 1 (5-HT1) receptors, particularly the 5-HT1D (also called 5-HT1Dalpha) and 5-HT1B (also called 5-HT1Dbeta) subtypes located on trigeminal sensory neurons innervating dural blood vessels. The 5-HT1B and 5-HT1D receptors function as autoreceptors, activation of which leads to inhibition of firing of serotonin neurons and a reduction in the synthesis and release of serotonin. Upon binding to these 5-HT1 receptor subtypes, sumatriptan inhibits adenylate cyclase activity via regulatory G proteins, increases intracellular calcium, and affects other intracellular events that lead to vasoconstriction and inhibition of sensory nociceptive (trigeminal) nerve firing and vasoactive neuropeptide release. Sumatriptan has the highest affinity for the 5-HT1D receptor, the most common serotonin receptor subtype in the brain, and a 2- to 17-fold lower affinity for 5-HT1A receptors; agonist activity at 5-HT1A and other serotonin receptors may be responsible for some of the adverse effects noted with administration of serotonin or serotonergic antimigraine drugs (eg, ergotamine, dihydroergotamine). Sumatriptan has essentially no affinity for (based on standard radioligand binding assays) nor pharmacologic activity at other serotonin receptors (eg, 5-HT2, 5-HT3) or at receptors of the dopamine1, dopamine2, muscarinic, histamine, benzodiazepine, or alpha1-, alpha2-, or beta-adrenergic type. Sumatriptan is a selective agonist of vascular serotonin (5-hydroxytryptamine; 5-HT) type 1-like receptors, probably the 5-HT1D and 5-HT1B subtypes. The mechanisms involved in the pathogenesis of migraine and cluster headache are not clearly understood; consequently, the precise mechanism of action of sumatriptan in the management of these disorders has not been established. However, current data suggest that sumatriptan may ameliorate migraine and cluster headache through selective constriction of certain large cranial blood vessels and/or inhibition of neurogenic inflammatory processes in the CNS. While some features of migraine clearly reflect effects on cerebral blood vessels, neurogenic mechanisms involving activation of the trigeminovascular system also have been implicated; current evidence suggests that both mechanisms may be involved. The vascular 5-HT1 receptor subtype that sumatriptan activates is present on cranial arteries in both dog and primate, on the human basilar artery, and in the vasculature of human dura mater and mediates vasoconstriction. This action in humans correlates with the relief of migraine headache. In addition to causing vasoconstriction, experimental data from animal studies show that sumatriptan also activates 5-HT1 receptors on peripheral terminals of the trigeminal nerve innervating cranial blood vessels. Such an action may also contribute to the antimigrainous effect of sumatriptan in humans. Sumatriptan selectively reduces carotid arterial blood flow and/or constricts carotid arteriovenous anastomoses in anesthetized animals without appreciable effects on arterial blood pressure or total peripheral resistance. The drug produces contraction of vascular smooth muscle in vitro in saphenous veins in dogs and humans, but such contractions are weaker than those produced by serotonin or ergot alkaloids (eg, methysergide). For more Mechanism of Action (Complete) data for Sumatriptan (9 total), please visit the HSDB record page. |
| Molecular Formula |
C18H27N3O6S
|
|
|---|---|---|
| Molecular Weight |
413.49
|
|
| Exact Mass |
341.14
|
|
| Elemental Analysis |
C, 52.29; H, 6.58; N, 10.16; O, 23.22; S, 7.75
|
|
| CAS # |
103628-48-4
|
|
| Related CAS # |
Sumatriptan; 103628-46-2; Sumatriptan hydrochloride; 103628-62-2; 143675-45-0 (hemisulfate)
|
|
| PubChem CID |
59772
|
|
| Appearance |
White to off-white solid powder
|
|
| Density |
1.2±0.1 g/cm3
|
|
| Boiling Point |
497.7ºC at 760 mmHg
|
|
| Melting Point |
165-166°C
|
|
| Flash Point |
254.8ºC
|
|
| Vapour Pressure |
1.24E-17mmHg at 25°C
|
|
| Index of Refraction |
1.552
|
|
| LogP |
0.34
|
|
| Hydrogen Bond Donor Count |
4
|
|
| Hydrogen Bond Acceptor Count |
8
|
|
| Rotatable Bond Count |
9
|
|
| Heavy Atom Count |
28
|
|
| Complexity |
498
|
|
| Defined Atom Stereocenter Count |
0
|
|
| SMILES |
S(C([H])([H])C1C([H])=C([H])C2=C(C=1[H])C(=C([H])N2[H])C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])[H])(N([H])C([H])([H])[H])(=O)=O.O([H])C(C([H])([H])C([H])([H])C(=O)O[H])=O
|
|
| InChi Key |
PORMUFZNYQJOEI-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C14H21N3O2S.C4H6O4/c1-15-20(18,19)10-11-4-5-14-13(8-11)12(9-16-14)6-7-17(2)3;5-3(6)1-2-4(7)8/h4-5,8-9,15-16H,6-7,10H2,1-3H3;1-2H2,(H,5,6)(H,7,8)
|
|
| Chemical Name |
butanedioic acid;1-[3-[2-(dimethylamino)ethyl]-1H-indol-5-yl]-N-methylmethanesulfonamide
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.05 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (6.05 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 2.5 mg/mL (6.05 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 4: 100 mg/mL (241.84 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4184 mL | 12.0922 mL | 24.1844 mL | |
| 5 mM | 0.4837 mL | 2.4184 mL | 4.8369 mL | |
| 10 mM | 0.2418 mL | 1.2092 mL | 2.4184 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00356603 | Completed | Drug: Sumatriptan Succinate | Migraine Disorders | GlaxoSmithKline | June 20, 2006 | Phase 3 |
| NCT01269281 | Completed | Drug: Sumatriptan | Healthy | Dr. Reddy's Laboratories Limited |
July 2005 | Phase 1 |
| NCT01507610 | Completed | Drug: Sumatriptan | Migraine | Optinose US Inc. | January 2012 | Phase 1 |
| NCT00847405 | Completed | Drug: Sumatriptan Succinate Drug: Imitrex® |
Healthy | Teva Pharmaceuticals USA | March 2003 | Phase 1 |
| NCT00846885 | Recruiting | Drug: Sumatriptan Succinate Drug: Imitrex® |
Healthy | Teva Pharmaceuticals USA | August 2004 | Phase 1 |
 |
|---|
 |