
| Size | Price | Stock | Qty |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g | |||
| Other Sizes |
Purity: =100%
| Targets |
serine/threonine kinase ; JNK1 (IC50 = 40 nM); JNK2 (IC50 = 40 nM); Aurora A (IC50 = 60 nM); TrkA (IC50 = 70 nM)
|
|---|---|
| ln Vitro |
SP600125 is originally characterized as a selective ATP-competitive inhibitor of c-Jun N-terminal kinase JNK. With an IC50 range of 5 to 10 mM, SP600125 prevents the phosphorylation of c-Jun in Jurkat T cells. With an IC50 of 5 μM to 12 μM, SP600125 inhibits the expression of inflammatory genes COX-2, IL-2, IL-10, IFN-γ, and TNF-α in CD4+ cells, such as Th0 cells isolated from either human cord or peripheral blood. It also blocks cell activation and differentiation. [1] Later research, however, has shown that SP600125 also inhibits aryl hydrocarbon receptor AhR) [2], Mps1 [3], and a panel of other serine/threonine kinases, including Aurora kinase A, FLT3, MELK, and TRKA[4]. In mouse beta cells, MIN6 (20 M) stimulates the phosphorylation of p38 MAPK and the activation of its downstream CREB-dependent promoter. [5] SP600125 (20 M) blocks the transition from the G2 phase to mitosis and causes endoreplication in HCT116 cells. This capability of SP600125 results from its inhibition of CDK1-cyclin B activation upstream of Aurora A and Polo-like kinase 1, not from its inhibition of JNK. [6]
SP600125, an anthrapyrazolone inhibitor of c-jun N-terminal kinase (JNK), has been used to characterize the role of JNK in apoptotic pathways. In this study, we have demonstrated an additional novel anti-apoptotic action of this inhibitor in MIN6 cells, a mouse beta cell line. SP600125 induced CREB-dependent promoter activation by 2.8-fold at 20 microM, the concentration at which it inhibited c-jun-dependent promoter activation by 51%. There was a significant (P<0.01) increase in CREB phosphorylation (serine 133) at 5 min, which persisted for a period of 2h. Examination of signaling pathways upstream of CREB showed a 2.5-fold increase in the active phospho form of p38 MAPK. This finding was further confirmed by an in vitro kinase assay using ATF-2 as substrate. SB203580, an inhibitor of p38 MAPK, partially blocked SP600125-mediated activation of CREB. These observations suggest that SP600125 could be used as a small molecular weight activator of CREB. [5] Cell cycle controls ensure that DNA replication (S phase) follows mitosis resulting in two precise copies of the genome. A failure of the control mechanisms can result in multiple rounds of DNA replication without cell division. In endoreplication, cells with replicated genomes bypass mitosis, then replicate their DNA again, resulting in polyploidy. Endoreplication from G2 phase lacks all hallmarks of mitosis. Using synchronized cells, we show that the c-Jun N-terminal kinase (JNK) inhibitor, SP600125, prevents the entry of cells into mitosis and leads to endoreplication of DNA from G2 phase. We show that cells proceed from G2 phase to replicate their DNA in the absence of mitosis. This effect of SP600125 is independent of its suppression of JNK activity. Instead, the inhibitory effect of SP600125 on mitotic entry predominantly occurs upstream of Aurora A kinase and Polo-like kinase 1, resulting in a failure to remove the inhibitory phosphorylation of Cdk1. Importantly, our results directly show that the inhibition of Cdk1 activity and the persistence of Cdk2 activity in G2 cells induces endoreplication without mitosis. Furthermore, endoreplication from G2 phase is independent of p53 control. [6] |
| ln Vivo |
SP600125 (15 mg/kg or 30 mg/kg) significantly reduces the expression of TNF- induced by lipopolysaccharide (LPS) and the apoptosis of CD4+ CD8+ thymocytes induced by anti-CD3 in mice. [1]
Because multiple cell-based assays confirmed the potent inhibition of TNF-α with SP600125, researchers proceeded to test SP600125 inhibitory activity in a mouse model of LPS-induced TNF-α expression. Mice were pretreated with SP600125 per os or i.v. administration and then challenged with bacterial endotoxin (LPS). Dexamethasone 21-acetate was used as a positive control. Administration of SP600125 at 15 or 30 mg/kg i.v. significantly inhibited TNF-α serum levels, whereas oral administration dose-dependently blocked TNF-α expression with significant inhibition observed at 30 mg/kg per os (Fig. 5a). Therefore, SP600125 demonstrates efficacy in an in vivo model of endotoxin-induced inflammation. [1] Genetic mutants have also revealed a role for JNK in the apoptotic cell death of immature T cells in the thymus. Compared with wild-type animals, JNK2 knock-out mice exhibited almost complete resistance to apoptosis of double-positive thymocytes 48 h after injection with CD3 Ab. Researchers repeated this study to observe the effect of the JNK inhibitor, SP600125 (Fig. 5b). In control animals, CD4+ CD8+ thymocytes represented just less than 40% of total thymocytes. Forty-eight hours after exposure to CD3 Ab in vivo, the percentage of CD4+ CD8 + cells had declined to 10%. Remarkably, mice receiving SP600125 showed almost complete resistance to CD3 Ab-mediated apoptosis with CD4+ CD8+ numbers the same as control animals. This study further demonstrated the in vivo efficacy of SP600125 and showed consistent data to that observed in JNK knock-out animals. [1] SP600125 attenuates LPS-induced ALI in rats in vivo [7] The lung W/D ratio was analyzed to evaluate pulmonary edema. The LPS-treated rats had higher W/D ratios compared with the control rats. However, the W/D ratio was significantly decreased following administration of SP600125 (Fig. 1A). Furthermore, the results from the ELISA demonstrated that the expression of TNF-α and IL-6 in the bronchoalveolar lavage fluid (BALF) in the LPS-treated rats was markedly increased compared with the rats in the control group. However, the expression levels of TNF-α and IL-6 in the BALF in rats in the SP600125 group were significantly decreased (Fig. 1B and C). To assess the pathological alterations, H&E staining was performed and the results revealed evidence of infiltration of inflammatory cells, interstitial edema and interalveolar septal thickening, as well as intra-alveolar and interstitial hemorrhage. However, following treatment with SP600125, the pathological changes in the lung tissues of the rats markedly decreased (Fig. 1D). These results demonstrated that SP600125 treatment alleviated LPS-induced ALI in vivo. Effect of SP600125 on claudin-4 expression and JNK phosphorylation in vivo [7] The results from the ELISA demonstrated that the expression of claudin-4 in BALF in the LPS-treated rats was markedly lower compared with the rats in the control group. However, the expression levels of claudin-4 in BALF in the rats from the SP600125 group were significantly increased (Fig. 3A). The mRNA and protein expression of claudin-4 was significantly reduced in rat lung tissues treated with LPS; however, this was reversed following administration of SP600125 (Fig. 3B–D). Western blot analysis demonstrated that JNK phosphorylation in lung tissues was significantly increased following LPS treatment. However, SP600125 administration led to a reduction in JNK phosphorylation in the lung tissues of LPS-induced ALI rats (Fig. 4A and B). |
| Enzyme Assay |
Based on the precise measurement of radioactive phosphotransfer to the substrate, SP600125's potency towards kinases such as MPS1, JNK, and Aurora kinase A is established. The optimal [ATP] (2Km) and [substrate] (5Km) concentrations are then used in each assay to determine the absolute Km values for ATP and the particular substrate for each enzyme. MPS1 activity is measured using 5 nM of MPS1 recombinant protein in 50 mM HEPES pH 7.5, 2.5 mM MgCl2, 1 mM MnCl2, 1 mM DTT, 3 μM NaVO3, 2 mM β-glycerophosphate, 0.2 mg/mL BSA, 200 μM P38-βtide substrate-peptide (KRQADEEMTGYVATRWYRAE), and 8 μM ATP with 1.5 nM 33P-γ-ATP. The IC50 of ten serial 1:3 dilutions of SP600125 (ranging from 30μM to 1.5 nM) is tested.
Biochemical Characterization of JNK Enzyme Activity.[1] Researchers have described in detail methods for the expression and purification of recombinant proteins, glutathione S-transferase-c-Jun, and JNK, along with methods for a complete kinetic evaluation. Double-reciprocal analysis was used to assess the kinetic mechanism. To obtain kinetic constants, the data were fit to the equation for a sequential mechanism by the nonlinear least-squares method of Cleland. ERK1 and p38–2 kinetic assays were identical to the JNK assay except in the choice of phosphoacceptor. The ERK assay measured the phosphorylation of myelin basic protein, and the p38–2 assay measured the phosphorylation of glutathione S-transferase-activating transcription factor (ATF). The time-resolved fluorescence assay for JNK has been described. |
| Cell Assay |
In 384 well plates, cells are sown. The cells are given SP600125 treatment for 72 hours after seeding, and the plates are then processed using a CellTiter-Glo assay. The IC50 value for proliferation is calculated after the inhibitory activity is assessed by comparing treated versus control data.
Use of SP600125 in Cell Culture.[1] SP600125 (molecular weight = 220) is poorly soluble in water. Stock solutions of at least 20 mM can be made by using 100% dimethyl sulfoxide. As a general guide, for every 10 μM SP600125, it is recommended to include 0.1% DMSO in the culture media (e.g., 30 μM/0.3% DMSO). Prewarming of media and the use of serum protein may enhance solubility. SP600125 typically precipitates as fine needles that are visible at 50-fold magnification. Cell culture and treatments [7] Human type II-like alveolar epithelial cells (A549) were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum, 2 mmol/l glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin, and maintained in a humid environment at 37°C and 5% CO2. The cells were then treated with LPS (10 μg/ml) and different concentrations of SP600125 (10, 20 and 40 nM). Following 24 h, the cells were collected for further analysis. Cell viability assay [7] Cell viability was evaluated using the Cell Counting kit (CCK)-8 assay. In brief, the cells were seeded into 96-well plates at a density of 3×103 cells/well and left to adhere overnight. The cells were then incubated with or without 0–40 nM SP600125. Then, 100 μl CCK-8 was added and incubated in the dark at 37°C for 3 h. The absorbance was determined using the MRX II microplate reader at a wavelength of 450 nm. |
| Animal Protocol |
Mice: Female CD-1 mice (8–10 weeks old) are administered a dose of SP600125 intravenously (IV) or orally (OS) in a PPCES vehicle (30% PEG-400/20% polypropylene glycol/15% Cremophor EL/5% ethanol/30% saline), 15 minutes before receiving an IV injection of LPS in saline (0.5 mg/kg). At 90 minutes, an abdominal vena cava terminal bleed is obtained, and the serum is recovered. Using an ELISA, samples are examined for mouse TNF-α.
Rats: The control group, the LPS group, the normal saline (NS) group, and the SP600125 group are formed from a total of 40 male Wistar rats (n=10). Intratracheal injection of LPS results in acute lung injury (ALI). 10 minutes after the LPS injection, normal saline or SP600125 is injected intraperitoneally (15 mg/kg). Animal Studies.[1] Mouse LPS/TNF assay was performed as follows: Female CD-1 mice (8–10 weeks of age) were dosed i.v. or per os with SP600125 in PPCES vehicle (30% PEG-400/20% polypropylene glycol/15% Cremophor EL/5% ethanol/30% saline), final volume of 5 ml/kg, 15 min before i.v. injection with LPS in saline (0.5 mg/kg; Escherichia coli 055:B5; Westphal method). At 90 min, a terminal bleed was obtained from the abdominal vena cava, and the serum was recovered. Samples were analyzed for mouse TNF-α by using an ELISA. [1] The in-life phase of the thymocyte apoptosis assay was performed in female C57BL/6 mice (Harlan, San Diego). SP600125 was administered at 0, 12, 24, and 36 h, 15 mg/kg s.c. in PPCES vehicle. Anti-CD3 (50 μg) i.p. (clone 145-2C11) was administered as a single dose immediately after SP600125 at time 0. After 48 h, mice were killed, and the thymus was dissected for thymocyte isolation. Treated and untreated mice thymuses were excised and immediately placed in complete medium (RPMI medium 1640 with 10% FBS, penicillin/streptomycin, and l-glutamine) on ice. Each thymus was then pressed between the frosted ends of 2 microscope slides to form a single cell suspension and collected through a 30 μm nylon mesh. Cells were stained for cell surface CD4 and CD8) and apoptosis and measured by flow cytometry. Model establishment[7] A total of 40 male Wistar rats were randomly divided into four groups (n=10): the control group, LPS group, normal saline group (NS) and the SP600125 group. ALI/Acute lung injury was induced via intratracheal injection of LPS as previously described. Briefly, the rats were anesthetized with pentobarbital sodium followed by intratracheal injection of 5 mg/kg LPS. The rats were then placed in a vertical position and rotated for 1 min to distribute the LPS in the lungs. Normal saline or SP600125 was administered via intraperitoneal injection (15 mg/kg) 10 min after the LPS injection. |
| References | |
| Additional Infomation |
Anthra[1,9-cd]pyrazol-6(2H)-one is a member of the class of anthrapyrazoles that is anthra[1,9-cd]pyrazole substituted at position 6 by an oxo group. An inhibitor of c-Jun N-terminal kinase. It has a role as a c-Jun N-terminal kinase inhibitor, a geroprotector and an antineoplastic agent. It is an anthrapyrazole, a cyclic ketone and an aromatic ketone.
Jun N-terminal kinase (JNK) is a stress-activated protein kinase that can be induced by inflammatory cytokines, bacterial endotoxin, osmotic shock, UV radiation, and hypoxia. We report the identification of an anthrapyrazolone series with significant inhibition of JNK1, -2, and -3 (K(i) = 0.19 microM). SP600125 is a reversible ATP-competitive inhibitor with >20-fold selectivity vs. a range of kinases and enzymes tested. In cells, SP600125 dose dependently inhibited the phosphorylation of c-Jun, the expression of inflammatory genes COX-2, IL-2, IFN-gamma, TNF-alpha, and prevented the activation and differentiation of primary human CD4 cell cultures. In animal studies, SP600125 blocked (bacterial) lipopolysaccharide-induced expression of tumor necrosis factor-alpha and inhibited anti-CD3-induced apoptosis of CD4(+) CD8(+) thymocytes. Our study supports targeting JNK as an important strategy in inflammatory disease, apoptotic cell death, and cancer.[1] Exposure of the immortalized human breast epithelial cell line MCF10A to the Jun N-terminal kinase (JNK) inhibitor anthra[1,9-cd]pyrazol-6(2H)-one (SP600125) suppressed, in a concentration-dependent manner (IC50 is approximately 2 microM), the induction of CYP1A1 by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Cotreatment with SP600125 also suppressed the accumulation of TCDD-induced nuclear aryl hydrocarbon receptor (AhR)-DNA complexes, as assessed by electrophoretic mobility shift assays. Concentrations of SP600125 < or = 50 microM did not transform the AhR into a DNA-binding species when added to rat liver cytosol. However, addition of SP600125 to cytosol just before TCDD addition completely suppressed AhR transformation and DNA binding (IC50 approximately 7 microM). Sucrose gradient analyses using rat liver and murine hepatoma 1c1c7 extracts demonstrated that SP600125 competed with TCDD for binding to the AhR. These results suggest that SP600125 is an AhR ligand and functions as an AhR antagonist at concentrations used to pharmacologically inhibit JNK.[2] The spindle assembly checkpoint ensures accurate chromosome segregation by delaying anaphase initiation until all chromosomes are properly attached to the mitotic spindle. Here, we show that the previously reported c-Jun amino-terminal kinase (JNK) inhibitor SP600125 effectively disrupts spindle checkpoint function in a JNK-independent fashion. SP600125 potently inhibits activity of the mitotic checkpoint kinase monopolar spindle 1 (Mps1) in vitro and triggers efficient progression through a mitotic arrest imposed by spindle poisons. Importantly, expression of an Mps1 mutant protein refractory to SP600125-mediated inhibition restores spindle checkpoint function in the presence of SP600125, showing that its mitotic phenotype is induced by Mps1 inhibition in vivo. Remarkably, primary human cells are largely resistant to the checkpoint-inactivating action of SP600125, suggesting the existence of Mps1-independent checkpoint pathways that are compromised in tumour cells.[3] Although in vitro studies have previously demonstrated that mitogen-activated protein kinases are important for the activation of transcription factors and the regulation of proinflammatory mediators, the function of c-Jun NH2-terminal kinase (JNK) in acute lung injury (ALI) remains to be fully elucidated. The present study aimed to investigate the effect of the JNK selective inhibitor SP600125 on lipopolysaccharide (LPS)-induced ALI. Pulmonary edema, the expression of inflammatory cytokines and pathological alterations were found to be significantly attenuated in LPS-induced ALI following treatment with SP600125 in vivo. In vitro, it was demonstrated that SP600125 administration significantly improved A549 cell viability in a dose-dependent manner using the Cell Counting kit-8 and the 5-ethynyl-2'-deoxyuridine incorporation assay. Furthermore, flow cytometric analysis demonstrated that the apoptotic rate was significantly reduced in a concentration-dependent manner following SP600125 injection. At the molecular level, SP600125 treatment dose-dependently inhibited JNK activation and upregulated claudin-4 expression in vivo and in vitro. In combination, the results from the present study indicated that the JNK inhibitor SP600125 protected against LPS-induced ALI in vivo and in vitro, possibly by upregulating the expression of claudin-4.[7] |
| Molecular Formula |
C14H8N2O
|
|
|---|---|---|
| Molecular Weight |
220.23
|
|
| Exact Mass |
220.063
|
|
| Elemental Analysis |
C, 76.35; H, 3.66; N, 12.72; O, 7.26
|
|
| CAS # |
129-56-6
|
|
| Related CAS # |
|
|
| PubChem CID |
8515
|
|
| Appearance |
Yellow to green solid powder
|
|
| Density |
1.5±0.1 g/cm3
|
|
| Boiling Point |
489.3±14.0 °C at 760 mmHg
|
|
| Melting Point |
281~282℃
|
|
| Flash Point |
246.8±26.5 °C
|
|
| Vapour Pressure |
0.0±1.2 mmHg at 25°C
|
|
| Index of Refraction |
1.799
|
|
| LogP |
3.18
|
|
| Hydrogen Bond Donor Count |
1
|
|
| Hydrogen Bond Acceptor Count |
2
|
|
| Rotatable Bond Count |
0
|
|
| Heavy Atom Count |
17
|
|
| Complexity |
343
|
|
| Defined Atom Stereocenter Count |
0
|
|
| SMILES |
O=C1C2=C3C(NN=C3C4=C1C=CC=C4)=CC=C2
|
|
| InChi Key |
ACPOUJIDANTYHO-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C14H8N2O/c17-14-9-5-2-1-4-8(9)13-12-10(14)6-3-7-11(12)15-16-13/h1-7H,(H,15,16)
|
|
| Chemical Name |
14,15-diazatetracyclo[7.6.1.02,7.013,16]hexadeca-1(15),2,4,6,9(16),10,12-heptaen-8-one
|
|
| Synonyms |
SP 600125; SP-600125; 129-56-6; 1,9-Pyrazoloanthrone; SP600125; Pyrazolanthrone; Dibenzo[cd,g]indazol-6(2H)-one; Pyrazoleanthrone; SP600125
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: 2.08 mg/mL (9.44 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 5% DMSO+corn oil: 5mg/mL View More
Solubility in Formulation 3: 1 mg/mL (4.54 mM) in Corn Oil (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication (<80°C). Solubility in Formulation 4: 3.33 mg/mL (15.12 mM) in 1% CMC-Na/saline water (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.5407 mL | 22.7035 mL | 45.4071 mL | |
| 5 mM | 0.9081 mL | 4.5407 mL | 9.0814 mL | |
| 10 mM | 0.4541 mL | 2.2704 mL | 4.5407 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
|
Effect of SP600125 administration on A549 cell viability and apoptosis.Exp Ther Med.2014 Jul;8(1):153-158 |
Effect of SP600125in vitroon the expression of claudin-4 and JNK phosphorylation.Exp Ther Med.2014 Jul;8(1):153-158 |
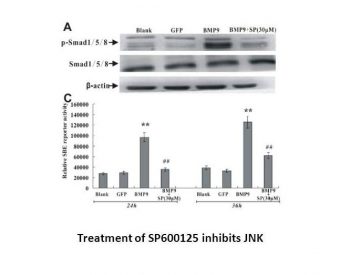 |
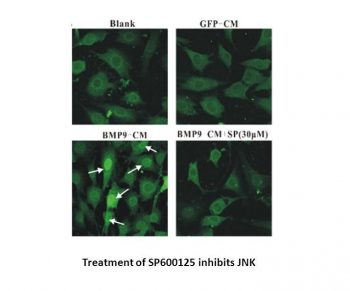 |
Effect of SP600125 on claudin-4 expression and JNK phosphorylationin vivo.Exp Ther Med.2014 Jul;8(1):153-158 |