
| Size | Price | Stock | Qty |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
Purity: ≥98%
SB525334 (SB-525334; SB 525334) is a potent and selective inhibitor of TGF-β (transforming growth factor-β) receptor I, ALK5 (activin receptor-like kinase) with potential anti-fibrotic activity. It inhibits TGF-β with an IC50 of 14.3 nM in a cell-free assay. SB525334 is 4-fold less potent to ALK4 than ALK5 and is inactive against ALK2, 3, and 6. In cell-based assays, SB-525334 at the concentration of 1 μM blocked TGF-β1-induced phosphorylation and nuclear translocation of Smad2/3 in renal proximal tubule cells and inhibited TGF-β1-induced increases in plasminogen activator inhibitor-1 (PAI-1) and procollagen alpha1(I) mRNA expression in A498 renal epithelial carcinoma cells.
| Targets |
ALK (IC50 = 14.3 nM)
|
|---|---|
| ln Vitro |
The study found that SB525334 (1 μM) reduces the proliferation of familial idiopathic pulmonary arterial hypertension (iPAH) pulmonary artery smooth muscle cells (PASMCs) with an IC50 of 295 nM. The effect is observed 15 minutes before stimulating with 0.625 ng/ml of TGF-β1, and evaluation is done after 6 days.
|
| ln Vivo |
In a rat model of pulmonary arterial hypertension (PAH), SB525334 (3-30 mg/kg; po; daily from days 17 to 35) dramatically reverses pulmonary arterial pressure[2].
In view of this profile, SB-525334 was used to investigate the role of TGF-beta1 in the acute puromycin aminonucleoside (PAN) rat model of renal disease, a model of nephritis-induced renal fibrosis. Orally administered doses of 1, 3, or 10 mg/kg/day SB-525334 for 11 days produced statistically significant reductions in renal PAI-1 mRNA. Also, the compound produced dose-dependent decreases in renal procollagen alpha1(I) and procollagen alpha1(III) mRNA, which reached statistical significance at the 10-mg/kg/day dose when compared with vehicle-treated PAN controls. Furthermore, PAN-induced proteinuria was significantly inhibited at the 10-mg/kg/day dose level. These results provide further evidence for the involvement of TGF-beta1 in the profibrotic changes that occur in the PAN model and for the first time, demonstrate the ability of a small molecule inhibitor of ALK5 to block several of the markers that are predictive of fibrosis and renal injury in this model.[1] We further demonstrate that SB525334 significantly reverses pulmonary arterial pressure and inhibits right ventricular hypertrophy in a rat model of PAH. Immunohistochemical studies confirmed a significant reduction in pulmonary arteriole muscularization induced by monocrotaline (used experimentally to induce PAH) after treatment of rats with SB525334. Collectively, these data are consistent with a role for the activin receptor-like kinase 5 in the progression of idiopathic PAH and imply that strategies to inhibit activin receptor-like kinase 5 signaling may have therapeutic benefit[2]. Blockade of TGF-beta signaling with the ALK5/type I TGF-beta R kinase inhibitor, SB-525334, was efficacious for uterine leiomyoma; significantly decreasing tumor incidence and multiplicity, and reducing the size of these mesenchymal tumors. However, SB-525334 was also mitogenic and antiapoptotic for epithelial cells in the kidney and exacerbated the growth of epithelial lesions present in the kidneys of these animals. Conclusion: Although pharmacologic inhibition of TGF-beta signaling with SB-525334 may be efficacious for mesenchymal tumors, inhibition of this signaling pathway seems to promote the development of epithelial tumors.[3] |
| Enzyme Assay |
SB-525334 (6-[2-tert-butyl-5-(6-methyl-pyridin-2-yl)-1H-imidazol-4-yl]-quinoxaline) has been characterized as a potent and selective inhibitor of the transforming growth factor-beta1 (TGF-beta1) receptor, activin receptor-like kinase (ALK5). The compound inhibited ALK5 kinase activity with an IC(50) of 14.3 nM and was approximately 4-fold less potent as an inhibitor of ALK4 (IC(50) = 58.5 nM). SB-525334 was inactive as an inhibitor of ALK2, ALK3, and ALK6 (IC(50) > 10,000 nM) [1].
|
| Cell Assay |
Cell Proliferation Assay[2]
Cell Types: PASMC cells Tested Concentrations: 1 μM Incubation Duration: Pre-incubated for 15 minutes (before stimulating with 0.625 ng/ml of TGF-β1), assessed after 6 days Experimental Results: Inhibited TGF-β1-mediated proliferation of familial iPAH PASMCs at an IC50 of 295 nM. |
| Animal Protocol |
Animal/Disease Models: Adult male SD (Sprague-Dawley) rats (MCT rat model of pulmonary hypertension)[2]
Doses: 3, 30 mg/kg Route of Administration: Oral administration; daily from days 17 to 35 Experimental Results: decreased the proportion of fully muscularized vessels to 28% at 3 mg/kg and returned fully muscularized vessel distribution beyond that seen at day 17 and approaching the phenotype observed in saline-exposed controls at 30 mg/kg. MCT Rat Model of Pulmonary Hypertension[2] Animals were housed at 24°C in a 12-hour light-dark cycle. Food and water were accessible ad libitum. The studies reported here conformed to the UK Animals (scientific procedures) Act 1986. MCT-induced PAH was performed as previously described.15 Briefly, adult male Sprague-Dawley rats (n = 10 per group) were anesthetized and subcutaneously injected with 40 mg/kg of MCT or sterile saline. Before commencement of dosing at day 17 the extent of hypertensive pathology was determined in animals (n = 5) per group via echocardiography. A further group of animals was also assessed via surgery and catheterization. SB-525334 compound was dosed orally (3 or 30 mg/kg) or vehicle alone was dosed daily until day 35, when the remaining animals were reassessed by echocardiography, surgery, and catheterization.[2] In vivo study. [3] The protocols involving the use of these rats were approved by the M.D. Anderson Cancer Center Institutional Animal Care and Use Committee. Animals were maintained on a 12 h light/dark cycle, with food and water provided ad libitum. To determine the effects of a TGF-β receptor inhibitor on uterine leiomyoma, female Eker rats 12 or 14 months old were given SB-525334 at a dose of 200 mg/L drinking water (estimated dose of 10 mg/kg/d) or received normal drinking water for 2 and 4 months. At 16 months of age, animals were sacrificed by CO2 asphyxiation and tissues were harvested and either snap-frozen in liquid nitrogen and stored at −80°C or fixed in 10% neutral buffered formalin and paraffin embedded. To further analyze the effects of SB-525334 on kidneys, 9-month-old male Eker rats were given plain drinking water or the compound in drinking water at 200 mg/L for 2 months. Rats were then sacrificed and tissues were harvested, fixed, and stored as described above. For histology, tissues were stained with H&E, and kidneys and multiple sections of female reproductive tract (uterus, vagina, and cervix) were examined microscopically by a pathologist blinded as to treatment group (see below). All tumors and proliferative lesions were identified and evaluated as previously described.[3] |
| References |
|
| Additional Infomation |
6-[2-tert-butyl-5-(6-methyl-2-pyridinyl)-1H-imidazol-4-yl]quinoxaline is a quinoxaline derivative.
SB-525334 (6-[2-tert-butyl-5-(6-methyl-pyridin-2-yl)-1H-imidazol-4-yl]-quinoxaline) has been characterized as a potent and selective inhibitor of the transforming growth factor-beta1 (TGF-beta1) receptor, activin receptor-like kinase (ALK5). The compound inhibited ALK5 kinase activity with an IC(50) of 14.3 nM and was approximately 4-fold less potent as an inhibitor of ALK4 (IC(50) = 58.5 nM). SB-525334 was inactive as an inhibitor of ALK2, ALK3, and ALK6 (IC(50) > 10,000 nM). In cell-based assays, SB-525334 (1 microM) blocked TGF-beta1-induced phosphorylation and nuclear translocation of Smad2/3 in renal proximal tubule cells and inhibited TGF-beta1-induced increases in plasminogen activator inhibitor-1 (PAI-1) and procollagen alpha1(I) mRNA expression in A498 renal epithelial carcinoma cells. In view of this profile, SB-525334 was used to investigate the role of TGF-beta1 in the acute puromycin aminonucleoside (PAN) rat model of renal disease, a model of nephritis-induced renal fibrosis. Orally administered doses of 1, 3, or 10 mg/kg/day SB-525334 for 11 days produced statistically significant reductions in renal PAI-1 mRNA. Also, the compound produced dose-dependent decreases in renal procollagen alpha1(I) and procollagen alpha1(III) mRNA, which reached statistical significance at the 10-mg/kg/day dose when compared with vehicle-treated PAN controls. Furthermore, PAN-induced proteinuria was significantly inhibited at the 10-mg/kg/day dose level. These results provide further evidence for the involvement of TGF-beta1 in the profibrotic changes that occur in the PAN model and for the first time, demonstrate the ability of a small molecule inhibitor of ALK5 to block several of the markers that are predictive of fibrosis and renal injury in this model.[1] Mutations in the gene for the transforming growth factor (TGF)-beta superfamily receptor, bone morphogenetic protein receptor II, underlie heritable forms of pulmonary arterial hypertension (PAH). Aberrant signaling via TGF-beta receptor I/activin receptor-like kinase 5 may be important for both the development and progression of PAH. We investigated the therapeutic potential of a well-characterized and potent activin receptor-like kinase 5 inhibitor, SB525334 [6-(2-tert-butyl-5-{6-methyl-pyridin-2-yl}-1H-imidazol-4-yl)-quinoxaline] for the treatment of PAH. In this study, we demonstrate that pulmonary artery smooth muscle cells from patients with familial forms of idiopathic PAH exhibit heightened sensitivity to TGF-beta1 in vitro, which can be attenuated after the administration of SB525334. We further demonstrate that SB525334 significantly reverses pulmonary arterial pressure and inhibits right ventricular hypertrophy in a rat model of PAH. Immunohistochemical studies confirmed a significant reduction in pulmonary arteriole muscularization induced by monocrotaline (used experimentally to induce PAH) after treatment of rats with SB525334. Collectively, these data are consistent with a role for the activin receptor-like kinase 5 in the progression of idiopathic PAH and imply that strategies to inhibit activin receptor-like kinase 5 signaling may have therapeutic benefit.[2] Purpose: Transforming growth factor beta (TGF-beta), which generally stimulates the growth of mesenchymally derived cells but inhibits the growth of epithelial cells, has been proposed as a possible target for cancer therapy. However, concerns have been raised that whereas inhibition of TGF-beta signaling could be efficacious for lesions in which TGF-beta promotes tumor development and/or progression, systemic pharmacologic blockade of this signaling pathway could also promote the growth of epithelial lesions. Experimental design: We examined the effect of a TGF-beta inhibitor on mesenchymal (leiomyoma) and epithelial (renal cell carcinoma) tumors in Eker rats, which are genetically predisposed to develop these tumors with a high frequency. Results: Blockade of TGF-beta signaling with the ALK5/type I TGF-beta R kinase inhibitor, SB-525334, was efficacious for uterine leiomyoma; significantly decreasing tumor incidence and multiplicity, and reducing the size of these mesenchymal tumors. However, SB-525334 was also mitogenic and antiapoptotic for epithelial cells in the kidney and exacerbated the growth of epithelial lesions present in the kidneys of these animals. Conclusion: Although pharmacologic inhibition of TGF-beta signaling with SB-525334 may be efficacious for mesenchymal tumors, inhibition of this signaling pathway seems to promote the development of epithelial tumors.[3] |
| Molecular Formula |
C21H21N5
|
|
|---|---|---|
| Molecular Weight |
343.42
|
|
| Exact Mass |
343.179
|
|
| Elemental Analysis |
C, 73.44; H, 6.16; N, 20.39
|
|
| CAS # |
356559-20-1
|
|
| Related CAS # |
|
|
| PubChem CID |
9967941
|
|
| Appearance |
Yellow to orange solid
|
|
| Density |
1.2±0.1 g/cm3
|
|
| Boiling Point |
540.5±45.0 °C at 760 mmHg
|
|
| Melting Point |
159 °C
|
|
| Flash Point |
238.6±21.7 °C
|
|
| Vapour Pressure |
0.0±1.4 mmHg at 25°C
|
|
| Index of Refraction |
1.636
|
|
| LogP |
4.05
|
|
| Hydrogen Bond Donor Count |
1
|
|
| Hydrogen Bond Acceptor Count |
4
|
|
| Rotatable Bond Count |
3
|
|
| Heavy Atom Count |
26
|
|
| Complexity |
476
|
|
| Defined Atom Stereocenter Count |
0
|
|
| SMILES |
N1([H])C(C2=C([H])C([H])=C([H])C(C([H])([H])[H])=N2)=C(C2C([H])=C([H])C3C(C=2[H])=NC([H])=C([H])N=3)N=C1C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H]
|
|
| InChi Key |
DKPQHFZUICCZHF-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C21H21N5/c1-13-6-5-7-16(24-13)19-18(25-20(26-19)21(2,3)4)14-8-9-15-17(12-14)23-11-10-22-15/h5-12H,1-4H3,(H,25,26)
|
|
| Chemical Name |
6-(2-(tert-butyl)-5-(6-methylpyridin-2-yl)-1H-imidazol-4-yl)quinoxaline
|
|
| Synonyms |
SB 525334; SB-525334; 6-(2-(tert-Butyl)-5-(6-methylpyridin-2-yl)-1H-imidazol-4-yl)quinoxaline; 6-[2-TERT-BUTYL-5-(6-METHYL-PYRIDIN-2-YL)-1H-IMIDAZOL-4-YL]-QUINOXALINE; MFCD11045307; 6-[2-tert-butyl-5-(6-methylpyridin-2-yl)-1H-imidazol-4-yl]quinoxaline; SB525334
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (7.28 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (7.28 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: 5% DMSO+corn oil:20 mg/mL |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9119 mL | 14.5594 mL | 29.1189 mL | |
| 5 mM | 0.5824 mL | 2.9119 mL | 5.8238 mL | |
| 10 mM | 0.2912 mL | 1.4559 mL | 2.9119 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
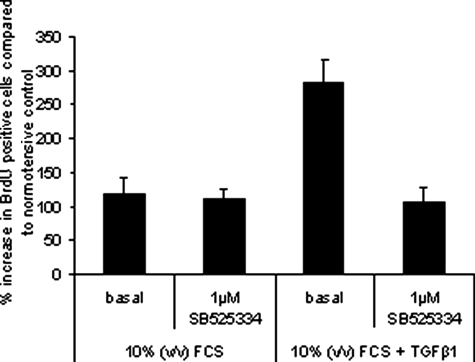 PASMCs derived from iPAH patients were plated at equal cell densities in 96-well plates.
Echocardiographic measurement of pulmonary hypertensive parameters in animals.Am J Pathol.2009 Feb;174(2):380-9. |
RV systolic pressure levels (A) and Fulton index measures (RV/LV + S weight ratio) (B) in rats exposed to MCT or saline-negative control.Am J Pathol.20 |