
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
Purity: ≥98%
SB505124 (SB-505124; SB 505124) is a novel, potent and selective inhibitor of TGFβR (TGF-β Receptor type I receptor) for ALK4, ALK5 (activin receptor-like kinase) with potential antineoplastic activity. It inhibits ALK4 and ALK5 with IC50s of 129 nM and 47 nM in cell-free assays, respectively. SB505124 also inhibits ALK7, but does not inhibit ALK1, 2, 3, or 6 and has potential anticancer activity. SB-505124 selectively and concentration-dependently inhibits ALK4-, ALK5-, and ALK 7-dependent activation of downstream cytoplasmic signal transducers, Smad2 and Smad3, and of TGF-beta-induced mitogen-activated protein kinase pathway components but does not alter ALK1, ALK2, ALK3 or ALK6-induced Smad signaling.
| Targets |
ALK4 (IC50 = 129 nM); ALK5 (IC50 = 47 nM); TGF-β Receptor type I receptors
|
|---|---|
| ln Vitro |
When SB-505124 is applied at concentrations up to 100 μM for 48 hours, renal epithelial A498 cells show no signs of toxicity. At dosages up to 10 μM, 505124 does not inhibit ALK2, but it does inhibit the closely related ALK4 with an IC50 value of 129±11 nM (about 2.5 times less sensitive than ALK5). In all three of these cell lines, TGF-β-induced phosphorylation of Smad2 is inhibited in a concentration-dependent manner by SB-505124 (1 μM). Despite the distinct patterns of activation in these cells, SB-505124 (1 or 5 μM) potently suppresses TGF-β-induced activation of JNK/SAP, extracellular signal-regulated kinase 1/2, and p38[1]. In vitro, SB-505124 (10 μM) inhibits CTGF and α-SMA expression, as well as Smad2 phosphorylation[2]. SB-505124 is shown to upregulate CTGF and α-SMA by immunofluorescence. Explants removed from eyes treated with SB-505124 during GFS exhibit robust cell outgrowth, but those treated with MMC exhibit weak outgrowth[3].
The gel was characterized for in vitro drug release and viscosity studies. Cytotoxicity of Pluronic® F-127 was examined by MTT assay using cultured rabbit subconjunctival fibroblasts. The in vitro drug release study demonstrated 100% drug release within 12 h. The gel did not show cytotoxicity to the cultured rabbit subconjunctival cells by MTT assay. [2] |
| ln Vivo |
When administered in combination with a single dose of carboplatin (60 mg/kg), SB-505124 (5 mg/kg; ip) does not provide any impact in C57Bl6 mice bearing A549 xenografts. However, in five of these animals, this combination produces long-lasting responses that do not require maintenance therapy[4].
The purpose of this study is to investigate a thermoreversible gel using Pluronic® F-127 to deliver an activin receptor-like kinase 5 (ALK-5) inhibitor SB-505124 in glaucoma filtration surgery (GFS). In addition, Pluronic® F-127 gel (18% w/v) containing 5 mg of SB-505124 was applied at the surgical site in an in vivo rabbit GFS model. In the in vitro viscosity study, the gel showed a change in viscosity (from 1000 cps to 45,000 cps) from low temperature (10°C) to body temperature (37°C). In the in vivo rabbit GFS model, the drug was successfully delivered by injection and no severe post-surgical complications were observed. A thermoreversible gel system with SB-505124 was successfully prepared and delivered for the rabbit GFS model, and it may provide a novel delivery system in GFS.[2] |
| Enzyme Assay |
Clinically, there is a great need for small molecule inhibitors that could control pathogenic effects of transforming growth factor (TGF-beta) and/or modulate effects of TGF-beta in normal responses. Inhibition of TGF-beta signaling would be predicted to enhance re-epithelialization of cutaneous wounds and reduce scarring fibrosis. Selective small molecule inhibitors of the TGF-beta signaling pathway developed for therapeutics will also be powerful tools in experimentally dissecting this complex pathway, especially its cross-talk with other signaling pathways. In this study, we characterized 2-(5-benzo[1,3]dioxol-5-yl-2-tert-butyl-3H-imidazol-4-yl)-6-methylpyridine hydrochloride (SB-505124), a member of a new class of small molecule inhibitors related to imidazole inhibitors of p38, which inhibit the TGF-beta type I receptor serine/threonine kinase known as activin receptor-like kinase (ALK) 5. We demonstrate that this compound selectively and concentration-dependently inhibits ALK4-, ALK5-, and ALK 7-dependent activation of downstream cytoplasmic signal transducers, Smad2 and Smad3, and of TGF-beta-induced mitogen-activated protein kinase pathway components but does not alter ALK1, ALK2, ALK3 or ALK6-induced Smad signaling. SB-505124 also blocks more complex endpoints of TGF-beta action, as evidenced by its ability to abrogate cell death caused by TGF-beta1 treatment. SB-505124 is three to five times more potent than a related ALK5 inhibitor described previously, SB-431542[1].
|
| Cell Assay |
An ALK-5 inhibitor, SB-505124, was used. A docking study was performed to investigate the interaction between the inhibitor and the receptor. Immunofluorescence for connective tissue growth factor (CTGF) and α-smooth muscle actin (α-SMA) was performed in cultured rabbit subconjunctival fibroblasts. Immunoblotting for phosphorylated Smad2 (pSmad2), CTGF, and α-SMA was also performed. In addition, cell outgrowth from dissected subconjunctival tissues placed in a cell culture flask with media was investigated[3].
|
| Animal Protocol |
Animal/Disease Models: C57Bl6 mice with A549 xenografts[4]
Doses: 5 mg/kg Route of Administration: Ip; daily Experimental Results: Had no effect alone, but administration with a single dose of carboplatin (60 mg/kg) resulted in durable responses without the need for maintenance therapy in five animals. In an in vivo rabbit GFS model, SB-505124 was delivered in a lactose tablet during surgery. Eyes were examined by slit-lamp and intraocular pressure (IOP) was measured until the time of bleb failure or up to 28 days after surgery. Tissue sections on day 5 after surgery were histologically evaluated after staining with hematoxylin and eosin. The sections were also immunostained for CTGF and α-SMA.[3] |
| References |
|
| Additional Infomation |
SB 505124 is a member of the class of imidazoles carrying tert-butyl, 1,3-benzodioxol-5-yl and 6-methylpyridin-2-yl substituents at positions 2, 4 and 5 respectively. It has a role as a TGFbeta receptor antagonist. It is a member of imidazoles, a benzodioxole and a member of methylpyridines.
|
| Molecular Formula |
C20H21N3O2
|
|
|---|---|---|
| Molecular Weight |
335.4
|
|
| Exact Mass |
335.163
|
|
| Elemental Analysis |
C, 71.62; H, 6.31; N, 12.53; O, 9.54
|
|
| CAS # |
694433-59-5
|
|
| Related CAS # |
356559-13-2
|
|
| PubChem CID |
9858940
|
|
| Appearance |
White to off-white solid powder
|
|
| Density |
1.2±0.1 g/cm3
|
|
| Boiling Point |
509.7±50.0 °C at 760 mmHg
|
|
| Flash Point |
180.5±20.4 °C
|
|
| Vapour Pressure |
0.0±1.3 mmHg at 25°C
|
|
| Index of Refraction |
1.600
|
|
| LogP |
4.77
|
|
| Hydrogen Bond Donor Count |
1
|
|
| Hydrogen Bond Acceptor Count |
4
|
|
| Rotatable Bond Count |
3
|
|
| Heavy Atom Count |
25
|
|
| Complexity |
466
|
|
| Defined Atom Stereocenter Count |
0
|
|
| InChi Key |
WGZOTBUYUFBEPZ-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C20H21N3O2/c1-12-6-5-7-14(21-12)18-17(22-19(23-18)20(2,3)4)13-8-9-15-16(10-13)25-11-24-15/h5-10H,11H2,1-4H3,(H,22,23)
|
|
| Chemical Name |
2-[4-(1,3-benzodioxol-5-yl)-2-tert-butyl-1H-imidazol-5-yl]-6-methylpyridine
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (7.45 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (7.45 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. View More
Solubility in Formulation 3: 30% PEG400+0.5% Tween80+5% propylene glycol:10 mg/mL |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9815 mL | 14.9076 mL | 29.8151 mL | |
| 5 mM | 0.5963 mL | 2.9815 mL | 5.9630 mL | |
| 10 mM | 0.2982 mL | 1.4908 mL | 2.9815 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
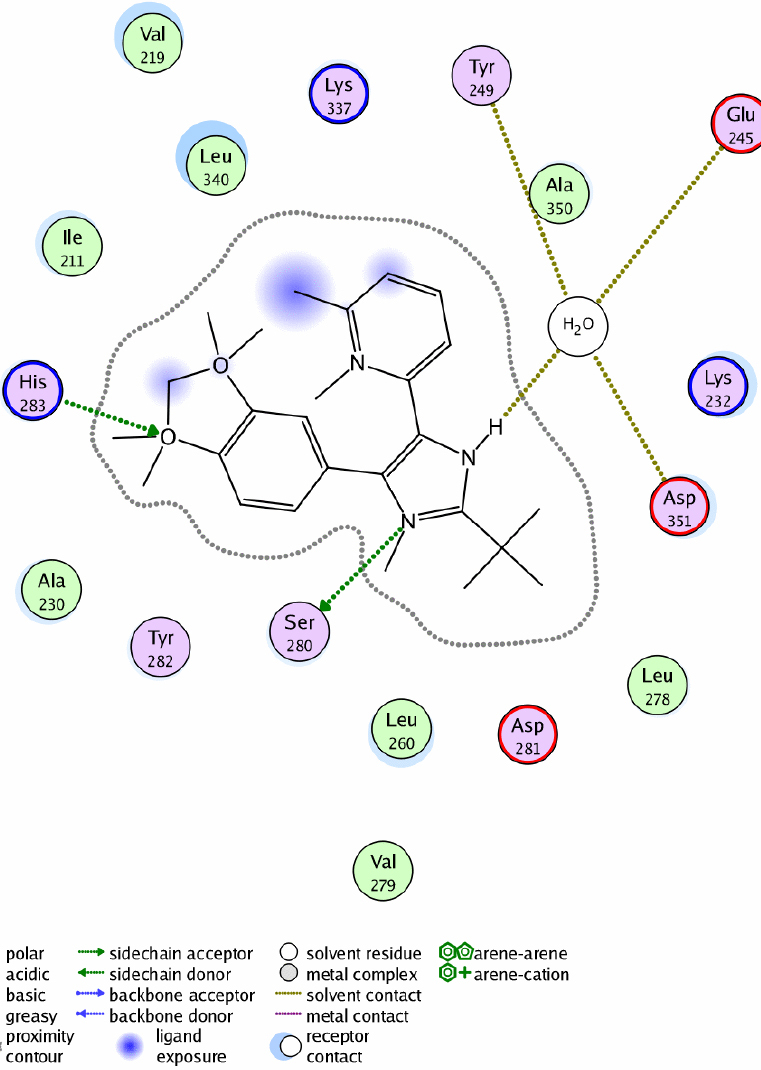 Docking results of SB-505124 (atom colors) in the ALK-5 ATP-binding pocket.Mol Vis.2010 Sep 16;16:1880-92. |
|---|
Effects of SB-505124 on cell density.Rabbit subconjunctival fibroblasts were incubated with 10 µM of SB-505124 or 0.04% MMC and then with or without TGF-β2 (2 ng/ml) in 12-well plates for 48 h (n=3).The number of cells was counted using a hemocytometer after trypsinization.Mol Vis.2010 Sep 16;16:1880-92. |
Western blotting for phosphorylated Smad2 (pSmad2), CTGF, and α-SMA.Rabbit subconjunctival fibroblasts were incubated with TGF-β2 and various concentrations of SB-505124. SB-505124 effectively reduced the pSmad2 level and the expression of CTGF and α-SMA induced by TGF-β2 in a concentration-dependent fashion.Mol Vis.2010 Sep 16;16:1880-92. |