
| Size | Price | Stock | Qty |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
Purity: ≥98%
Saracatinib (formerly also known as AZD-0530; AZD0530) is a novel, potent Src kinase inhibitor with potential antineoplastic activity. It inhibits Src with an IC50 of 2.7 nM in cell-free assays, and also potently inhibits other kinases such as c-Yes, Fyn, Lyn, Blk, Fgr and Lck; Saracatinib is less active against Abl and EGFR (L858R and L861Q). Saracatinib exhibits excellent in vivo antitumor efficacy in various tumors such as Src3T3 allografts, DU145, Calu-6, MDA-MB-231, AsPc-1 and BT474C xenografts. It has been reported to inhibit Src activation in DU145 and PC3 cell lines (prostate cancer cell lines). Both of c-Myc and cyclin D1 expression are decreased by Saracatinib. Saracatinib can inhibit the ERK1/2 and GSK3b phosphorylation as well as decrease β-catenin level in cells. Saracatinib inhibits the prostate tumor cell growth by inducing cycle arrest at G1/S phase. Saracatinib dose-dependently blocks cell migration in DU145 and PC3 cell lines.
| Targets |
Src (IC50 = 2.7 nM); v-Abl (IC50 = 30 nM); EGFR (IC50 = 66 nM); c-Kit (IC50 = 200 nM)
|
||
|---|---|---|---|
| ln Vitro |
An oral Src inhibitor called Saracatinib (AZD0530) has been shown to have strong antimigratory and anti-invasive properties in vitro. It also prevents bladder cancer metastases in a mouse model. Saracatinib's antiproliferative efficacy varies between cell lines (IC50 0.2-10 μM). Saracatinib exhibits varying antiproliferative efficacy in a variety of human cancer cell lines expressing endogenous Src and potently inhibits the proliferation of Src3T3 murine fibroblasts. IC50 values of 0.2-0.7 μM are obtained for five human cancer cell lines examined with Saracatinib, representing the tumor types colon, prostate, lung, and leukemia, indicating submicromolar growth suppression. Saracatinib suppresses the Bcr-Abl-driven human leukemia cell line K562's proliferation in 3-day MTS cell proliferation tests, with an IC50 of 0.22 μM. Saracatinib decreases human lung cancer A549 cells' movement in the microdroplet migration assay in a concentration-dependent manner (IC50 0.14 μM)[1].
Inhibition of isolated protein kinase activity [1] Saracatinib (AZD0530) potently inhibited Src and the other Src tyrosine kinase family members investigated (c‐Yes, Fyn, Lyn, Blk, Fgr, and Lck), with high selectivity observed against a panel of other protein kinases involved in signal transduction (Table 1), including Csk, the intracellular negative regulator of Src activation. The only other notable activities observed were versus Abl (in common with other ATP‐competitive Src inhibitors (Golas et al., 2003; Lombardo et al., 2004)) and versus activating mutant forms of the EGFR (L858R and L861Q). Inhibition of cell proliferation [1] Saracatinib (AZD0530) potently inhibited the in vitro proliferation of Src3T3 mouse fibroblasts and demonstrated variable antiproliferative activity in a range of human cancer cell lines containing endogenous Src (Table 2). Sub micromolar growth inhibition of five of the human cancer cell lines tested with AZD0530 (tumor types: colon, prostate, lung, and leukemia) was observed with IC50 values of 0.2–0.7μM. In 3‐day MTS cell proliferation assays (Promega G3580), AZD0530 inhibited in vitro proliferation of the Bcr–Abl‐driven human leukemia cell line K562 with an IC50 of 0.22μM. Inhibition of cell migration [1] In the microdroplet migration assay, Saracatinib (AZD0530) reduced the migration of human lung cancer A549 cells in a concentration‐dependent manner (IC50 0.14μM; Figure 1A). AZD0530 at concentrations of 0.01–0.5μM demonstrated a clear dose‐dependent antimigratory effect compared with untreated controls in monolayer scratch assays of human breast cancer MDA‐MB‐231 cells (Figure 1B). AZD0530 also dose dependently inhibited the EGF‐ and collagen‐stimulated migration of NBT‐II bladder cancer cells (Figure 1C). Moreover, at a single concentration of 0.25μM, AZD0530 consistently inhibited migration in monolayer scratch assays of bladder cancer cell lines (31%, 37%, and 78% inhibition versus untreated controls in T24, SCaBER, and 1A6 cell lines, respectively). Inhibition of cell invasion [1] Saracatinib (AZD0530) treatment significantly impaired the invasion of HT1080 cells through a 3‐dimensional (3D) collagen matrix (Figure 1D). In contrast to other 3D invasion assays, our assay (see Methods) monitors the proportion of total cells within a 3D matrix invading beyond a specified depth (60μm), thereby distinguishing repressed cellular invasion from cell loss that may be due to impaired adhesion, proliferation, or excessive cell death. This method confirms that AZD0530 exposure produces a significant and dose‐dependent inhibition of the invasive properties of HT1080 cells. Inhibition of cell scattering [1] Saracatinib (AZD0530) 1μM completely inhibited EGF‐induced cell scattering in NBT‐II bladder cancer cells, restoring cell–cell adhesion and the localization of desmoplakin to desmosomal junctions (Figure 2A). Inhibition of paxillin phosphorylation [1] Saracatinib (AZD0530) dose dependently inhibited phosphorylation of the Src substrate paxillin in NBT‐II bladder cancer cells (Figure 2B). Moreover, AZD0530 treatment (1μM) induced a relocalization of paxillin from the cell membrane into the cell cytoplasm (Figure 2C) further supporting a role for Src in the early development of cell migratory behavior. Effects on the EGFR [1] Given the moderate inhibitory effect observed for Saracatinib (AZD0530) against the EGFR in the isolated kinase assay (IC50 66nM; Table 1), further studies were performed to investigate AZD0530 effects on the EGFR. In a head‐to‐head assay measuring EGFR phosphorylation in KB cells, AZD0530 exhibited an IC50 of 1.25μM compared with 11nM for gefitinib, an EGFR tyrosine kinase inhibitor. In isolated kinase assays, AZD0530 exhibited IC50s of 5 and 4nM respectively, for EGFR point‐mutant isoforms L858R (Millipore 14‐626) and L861Q (Millipore 14‐627). Effects on the EGFR L858R isoform were further investigated in PC‐9 cells, a non‐small‐cell lung cancer (NSCLC) cell line harboring this EGFR mutation. AZD0530 inhibited growth of PC‐9 cells with an IC50 of 0.23μM in 3‐day MTS cell proliferation assays (Promega G3580). Enzyme kinetics [1] Pre‐incubation of Src kinase with 100nM Saracatinib (AZD0530) (in the presence of 160μM ATP) resulted in >95% inhibition of Src activity. A subsequent 10‐fold dilution to 10nM AZD0530 in the presence of a high concentration of ATP (1.60mM) led to a regain of Src activity, with only 30% inhibition, suggesting that inhibition was reversible. A similar degree of inhibition was observed for the same final concentrations of AZD0530 and ATP without the dilution step. The reversibility studies implied that Saracatinib (AZD0530) was competitive with ATP, as has been reported for other anilinoquinazolines (Bridges, 2001). ATP‐competitive inhibition was confirmed in experiments where both the concentration of ATP and that of AZD0530 were varied at fixed concentrations of the substrate, Src II peptide. The estimated inhibition constant, K is was 1.5 and the 95% confidence interval (CI) was 1.2, 1.9nM. This is lower than the IC50 of 2.7nM reported in Table 1 because it relates to a situation in the absence of competing ATP. AZD0530 was shown to follow pure noncompetitive kinetics when the concentration of Src II peptide was varied at 250μM ATP, with an apparent K i′ of 10nM (95% CI 8.4, 12.0; Figure 3A and B), which corresponds to a K i of 1.1nM (95% CI 0.9, 1.3) in the absence of ATP. Many kinases have structural differences between their active and inactive forms (Huse and Kuriyan, 2002). Inactivated Src adopts a closed conformation and AZD0530 binds approximately 10‐fold more weakly to inactive Src (K d 11nM; 95% CI 9.3, 14). Dual c-Met and Src inhibition is more effective than either inhibitor alone [2] MD-MSCs treated with saracatinib (0.5 μmol/L) together with increasing cabozantinib concentrations had a three-fold decrease in the IG50 of cabozantinib (0.6 μmol/L, compared with 2.2 μmol/L for cabozantinib alone), and more than five-fold selectivity over WT-MSCs (IG50 = 3.4 μmol/L; Fig. 4A). Treatment of MD-MSCs with Saracatinib (AZD0530) (0.5 μmol/L) and increasing cabozantinib concentration resulted in decreased phosphorylation levels of c-Met(Y1234/1235), Erk1/2, and Akt(T308) after 6 hours (Fig. 4B). After 24 hours of the combination treatment, MD-MSCs had decreased cyclin D1 levels and increased p27 levels at lower cabozantinib concentrations than those treated with cabozantinib alone (Fig. 4C). Dual c-Met and Src inhibition reduces viability of primary VS cells [2] We investigated the efficacy of this drug combination in decreasing viability of primary VS cells obtained from two patients. VS01 was a sporadic VS with a heterozygous duplication of NF2 exon 5 and a homozygous duplication of exon 7. VS02 was from an NF2 patient with an exon 14 deletion. VS cells were cultured with cabozantinib (2 μmol/L) and Saracatinib (AZD0530) (2 μmol/L), alone or in combination, for 48 hours. For both VS01 and VS02, the combination treatment reduced VS cell viability by approximately 35%–40% compared with vehicle (0.3% DMSO) and was significantly more effective than saracatinib alone. In addition, for VS01 cells, the combination treatment was more effective than cabozantinib alone (Fig. 4D). |
||
| ln Vivo |
Treatment with Saracatinib (AZD0530) potently and dose-dependently suppresses the proliferation of transplanted Src3T3 fibroblasts under the skin in mice and rats. Significant tumor growth inhibition is observed in both models at doses ≥6 mg/kg/day (60% in mice and 98% in rats versus animals treated with vehicle), and complete tumor growth inhibition is observed at the highest doses investigated (100% in rats and mice at 25 mg/kg/day and 10 mg/kg/day, respectively) [1].
Src3T3 allografts and xenografts [1] Saracatinib (AZD0530) treatment potently inhibited the proliferation of subcutaneously transplanted Src3T3 fibroblasts in mice (Figure 4B) and rats (Hennequin et al., 2006) in a dose‐dependent manner. In both models, significant inhibition of tumor growth was seen at doses ≥6mg/kg/day (60% inhibition in mice [P<0.01] and 98% inhibition in rats [P<0.001] versus animals treated with vehicle) and, at the maximum doses investigated, complete tumor growth inhibition was observed (100% inhibition at 25mg/kg/day in mice and 10mg/kg/day in rats) (Hennequin et al., 2006). Human tumor xenografts [1] Once‐daily oral dosing of Saracatinib (AZD0530) resulted in a moderate growth delay in 4/10 xenograft models tested (Table 3). In the remainder of the xenograft panel, AZD0530 treatment failed to inhibit primary tumor growth. Representative xenograft growth delay curves for an AZD0530 growth‐inhibition‐sensitive (Calu‐6) and an AZD0530 growth‐inhibition‐insensitive model (LoVo) are shown in Figure 4C and D, respectively. AZD0530 did not affect any aspect of tumor vascularity in xenograft models as assessed by blood vessel number or CD31 positive vessels (data not shown). NBT‐II bladder cancer metastasis model [1] In nude mice bearing subcutaneous xenografts of NBT‐II bladder cancer cells, once‐daily Saracatinib (AZD0530) treatment at doses of 10, 25, and 50mg/kg/day resulted in a small, non significant delay in xenograft growth at the highest dose only (50mg/kg/day; Figure 4E). In contrast, all three doses of AZD0530 resulted in a reduction in the number of mice from which tumor colonies could be grown from mesenteric lymph node extracts (Figure 5A). In an additional experiment, this antimetastatic effect of AZD0530 did not appear to be affected by a 7‐day delay between cell inoculation and drug administration. One out of seven mice developed metastases in a group that started treatment with AZD0530 50mg/kg/day 7 days after tumor cell inoculation, and 1/7 developed metastases in a group that started treatment with the same dose immediately after cell inoculation. Phosphorylation of Src kinase substrates FAK and paxillin [1] Levels of phosphorylated FAK (pY861) and paxillin (pY31), assessed by immunohistochemistry using phosphospecific antibodies, were reduced markedly in tumors from animals bearing both growth‐inhibition‐sensitive and growth‐inhibition‐insensitive xenografts after 14–28 days' treatment with Saracatinib (AZD0530) 50mg/kg/day (Figure 5B–D). Antibodies detecting non‐phospho epitopes of FAK or paxillin showed no effect on the expression of either protein (data not shown). Dual c-Met and Src inhibition slows MD-MSC growth in vivo [2] To evaluate the efficacy of cabozantinib and Saracatinib (AZD0530) in vivo, luciferase-expressing MD-MSCs were grafted into the sciatic nerves of NSG mice, and the graft size was monitored by bioluminescence imaging (BLI). Upon confirmation of successful grafting (Supplementary Fig. S2A), mice were assigned into vehicle, cabozantinib (12.5 mg/kg/day), saracatinib (25 mg/kg/day), and combination (cabozantinib and saracatinib at 12.5 mg/kg/day and 25 mg/kg/day, respectively) treatment cohorts. BLI showed that grafts in the combination-treated group had a significantly slower growth rate compared with those in the single-agent groups (Fig. 5A and B). Although the vehicle-treated allografts had a 160-fold increase in BL signal over 14 days, the grafts treated with saracatinib or cabozantinib had a 50- and 60-fold increase in BL signal, respectively. Significantly, the allografts from the combination group had only a 25-fold increase in BL signal after 14 days of treatment (Fig. 5C). The reduction in BL signals correlated with lower tumor weights in the combination-treated group compared with the vehicle group (Fig. 5D; Supplementary Fig. S2B). Dual c-Met and Src inhibition modulates FAK and ERK signaling pathways in allografts [2] To assess the signaling pathways modulated by drug treatments, allograft sections were analyzed by immunohistochemistry. Similar to the in vitro findings, Saracatinib (AZD0530) decreased the FAK phosphorylation levels, and cabozantinib decreased the ERK1/2 phosphorylation levels compared with vehicle controls (Fig. 5E). These changes were also observed in the combination-treated allografts. Moreover, CD31 staining of endothelial cells was decreased in grafts treated with cabozantinib compared with vehicle controls, indicating that cabozantinib targets the vasculature in vivo as well (Fig. 5E). Grafts from mice treated with cabozantinib or saracatinib, alone or in combination, had fewer cyclin D1 and Ki67-positive cells compared with those from vehicle-treated mice. In addition, grafts in the combination-treated group had elevated cleaved caspase 3 staining, compared with those in the vehicle and single treatment groups (Fig. 5E), supporting the conclusion that the drug combination induces apoptosis of MD-MSCs in vivo. Dual c-Met and Src inhibition induces MD-MSC apoptosis [2] To confirm the in vivo findings, we studied caspase-dependent apoptosis in cultured MD-MSCs. Caspase 3/7 activity was triggered by cabozantinib or Saracatinib (AZD0530) alone only when administered at high doses (3 μmol/L saracatinib or 10 μmol/L cabozantinib) for 19 to 24 hours (Fig. 6A). However, cotreatment of MD-MSCs with saracatinib (0.5 μmol/L) and increasing cabozantinib concentrations induced caspase 3/7 activity at 100-fold lower concentrations of cabozantinib compared with cabozantinib administered alone (0.1 μmol/L vs. 10 μmol/L; Fig. 6A). Similarly, MD-MSCs treated with cabozantinib (0.5 μmol/L) and increasing saracatinib concentrations induced caspase 3/7 activity at approximately 30-fold lower saracatinib concentrations compared with saracatinib administered alone (0.1 μmol/L vs. 3 μmol/L; Fig. 6A). A membrane asymmetry assay confirmed that the drug combination induced a larger apoptotic cell population than either drug alone (Fig. 6B). Treatment with 2 μmol/L saracatinib resulted in 6.2% apoptotic cells, 4.8% dead cells, and 88.4% live cells, whereas treatment with 2 μmol/L cabozantinib had 7.7% apoptotic cells, 9% dead cells, and 82.8% live cells. The combination treatment increased the apoptotic population to 20% after 19 hours (Fig. 6C). When administered alone, saracatinib did not induce cleavage of caspase 3. Cabozantinib induced caspase cleavage at 1μmol/L whereas in the presence of 0.5 μmol/L saracatinib, caspase cleavage was detected at 0.1 μmol/L cabozantinib (Fig. 6D). Collectively, our results indicate that although the individual drugs promote a G1 cell-cycle arrest of MD-MSCs, saracatinib and cabozantinib combination is cytotoxic and promotes caspase 3/7-dependent apoptosis. |
||
| Enzyme Assay |
Isolated protein kinase assay [1]
Inhibition of tyrosine kinase activity was examined using an enzyme‐linked immunosorbent assay (ELISA) with recombinant catalytic domains of a panel of receptor and non‐receptor tyrosine kinases (in some cases only part of the catalytic domain was used). This method has been described previously (Plé et al., 2004). Saracatinib (AZD0530) dose ranges varied depending on the activity versus the particular kinase tested, but were typically 0.001–10μM. Specificity assays against a panel of serine/threonine kinases were performed using a filter capture assay with 32P. Briefly, multidrop 384 plates containing 0.5μL Saracatinib (AZD0530) or controls (dimethyl sulfoxide [DMSO] alone or pH 3.0 buffer controls) were incubated with 15μL of enzyme plus peptide/protein substrate for 5min before the reaction was initiated by the addition of 10μL of 20mM Mg.ATP. For all enzymes the final concentration was approximated to the Michaelis constant (K m). Assays were carried out for 30min at room temperature before termination by the addition of 5μL orthophosphoric acid. After mixing, the well contents were harvested onto a P81 Unifilter plate, using orthophosphoric acid as the wash buffer. Microcal Origin software was used to interpolate IC50 values by nonlinear regression. Enzyme kinetics [1] Investigation of the reversibility and the mechanism of Saracatinib (AZD0530) inhibition was conducted using a full‐length activated human Src (phosphorylated at tyrosine 416, Upstate Biotech, Dundee, UK) in a continuous, coupled assay adapted from Jenkins (1991). ATP and peptide substrate (Src II peptide) concentrations were varied in turn (ATP 40–1280μM; Src II peptide 100–800μM), in conjunction with AZD0530 (0–30nM), at saturating concentrations of the non‐varied substrate (ATP 1.6mM; Src II peptide 1.0mM). The binding affinity of AZD0530 for inactivated Src (phosphorylated at tyrosine 527, not tyrosine 416) was measured using a BIAcore inhibition‐in‐solution assay (Karlsson et al., 2000). The assay followed competition binding between AZD0530 and an immobilized ureidoquinazoline (Sullivan et al., 2005) for binding to Src. Data analysis was performed by unweighted nonlinear regression using GraFit, version 5 and an F‐test (Mannervik, 1982) was used to identify the most suitable equation. |
||
| Cell Assay |
Cell lines [1]
The cell lines used were mouse NIH 3T3 fibroblasts engineered to overexpress a constitutively active form of human Src (Src3T3) with a point mutation at the negative regulatory tyrosine site in the c‐terminal domain (Y530F) (Plé et al., 2004) and human cancer cell lines containing endogenous Src (Table 2). Src3T3 fibroblasts form colonies in soft agar and grow subcutaneously in immunocompromised athymic rats and mice in vivo, while the wild‐type parental 3T3 cells do not. Src3T3 cells will grow in medium containing as little as 0.5% fetal calf serum (FCS; used in the assay conditions), whereas wild‐type non‐Src‐transfected 3T3 cells will not grow in these low serum conditions. AZD0530 functional activity against Abl kinase was assessed in an in vitro proliferation assay using K562 human leukemia cells known to be growth driven through Bcr–Abl activity (Lozzio and Lozzio, 1975). NBT‐II rat bladder cancer cells were sourced and cultured as described previously (Boyer et al., 2002). Cell proliferation assay [1] Cell proliferation was assessed using a colorimetric 5‐bromo‐2′‐deoxyuridine (BrdU) Cell Proliferation ELISA kit (Roche Diagnostics GmbH), as described previously (Plé et al., 2004). Briefly, cells were plated onto 96‐well plates (1.5×104cells/well), the following day 0.039–20μM Saracatinib (AZD0530) in DMSO (at a final concentration of 0.5%) was added and the cells were incubated for 24h. The cells were pulse labeled with BrdU for 2h and fixed. Cellular DNA was then denatured with the provided solution and incubated with antiBrdU peroxidase for 90min. Following three washes with phosphate‐buffered saline, tetramethylbenzidine substrate solution was added and the plates were incubated on a plate shaker for 10–30min until the positive control absorbance at 690nm was approximately 1.5 absorbance units. EGFR phosphorylation assay [1] KB (nasopharyngeal carcinoma) cells were seeded at 5000cells/well in 96‐well plates and cultured for 72h in Rosewell Park Memorial Institute (RPMI) 1640 media with 10% FCS, followed by 24h' incubation with serum‐free RPMI 1640. Cells were treated with compound for 90min at concentrations ranging from 0 to 10μM. Cells were incubated with 15ng/mL EGF ligand (concentration required to increase receptor phosphorylation to 90% of maximum) for 5 min prior to lysis. Levels of phosphorylated EGFR were measured using the human phospho‐EGFR Duoset ELISA kit. Microdroplet migration (chemokinesis) assay [1] The microdroplet migration assay assesses the ability of test compounds to inhibit the random motility (chemokinesis) of human epithelial A549 lung cancer cells, which were routinely cultured in Dulbecco's modified Eagle's medium (DMEM)+10% FCS. The assay method has been described in full previously (Plé et al., 2004). Briefly, A549 cells (2×107/mL) were suspended in warm DMEM (37°C) containing 0.3% agarose. The suspension was pipetted into 96‐well plates (2μL/well) and chilled briefly on ice to allow the agarose microdroplet to gel. When set, 90μL of chilled RPMI 1640 medium was added to each well, followed by 10μL Saracatinib (AZD0530) (0.02–5μM). The plates were incubated for 72h at 37°C to allow migration to occur. Cell migration was measured by taking several equidistant measurements from around the agar droplet to the migrating front of the motile cells. An illustration of the microdroplet migration assay is shown in Figure 1A, indicating how cell migration was measured. Monolayer scratch assay [1] Human MDA‐MB‐231 breast cancer cells and T24, SCaBER, and 1A6 bladder cancer cells were grown as monolayers in adapted six‐well tissue culture plates. Confluent monolayers were gently scraped with a sterile pipette tip to form a scratch and Saracatinib (AZD0530) (0.01–0.5μM) was added to the wells in medium (phenol red‐free DMEM supplemented with 10% charcoal/dextran‐treated FCS and 1% l‐glutamine). Cell movement back into the area of the scratch was recorded by time‐lapse photography over 18h. NBT‐II cell adhesion and migration assays [1] The effects of Saracatinib (AZD0530) on EGF‐ and collagen‐induced motility of NBT‐II cells were assessed by videomicroscopy as described previously (Valles et al., 2004). The effect of AZD0530 on EGF‐induced cell dispersion was evaluated by immunofluorescence with anti‐desmoplakin antibodies to monitor the EGF‐induced loss of desmosomes from the cell periphery, as reported before (Boyer et al., 1997). Assessment of paxillin phosphorylation [1] The effects of Saracatinib (AZD0530) on paxillin phosphorylation was assessed in NBT‐II cells that had been stimulated with 100ng/mL EGF for 15min in the presence of increasing amounts of Saracatinib (AZD0530). Paxillin tyrosine phosphorylation was revealed by immunoblotting with polyclonal antibodies against phospho Y118‐paxillin and total paxillin. 3D invasion assay [1] Fibrillar collagen type I gels (1mg/mL final concentration) were prepared by neutralizing a solution of collagen type I with 1/10 volume 10× DMEM concentrate, diluted to a final concentration of 1× with distilled H2O, to which 1/11 volume 0.1N NaOH was added. Fibrillar collagen gels (80μL) were set within the upper chamber of a 24‐well transwell insert, above an 8μm pore‐size polycarbonate filter (Costar, Corning Inc.) at 37°C for 18h prior to cell seeding. Human HT1080 fibrosarcoma cells (ATCC CCL 121; DMEM, supplemented with 0.2% FCS and 2mM l‐glutamine) were seeded on top of a collagen gel in an upper transwell chamber (1×104cells per transwell). DMEM supplemented with 10% FCS and 2mM l‐glutamine (750μL total volume) was placed in the lower chamber to provide a chemotactic gradient. Saracatinib (AZD0530) (0.1, 1, and 10μM) or vehicle (0.1% DMSO) was added to the cells prior to seeding and also to media within the lower chamber. Transwells were incubated at 37°C for 72h and then incubated with 10μM Hoechst 33342 in serum‐free DMEM for 30min at 37°C. Invading cells were visualized by confocal microscopic analysis using a Bio‐Rad Radiance 2000 multiphoton confocal illumination unit attached to a Nikon Eclipse inverted microscope. Quantification of cell invasion was performed with modification to the protocol as described previously (Carragher et al., 2006). Briefly, using a 20× objective, optical sections were scanned at 20μm intervals from the collagen gel surface. Image‐Pro analysis software was applied to identify the number of positive pixels (Hoechst stain above a threshold) and segment into individual nuclei per optical section. The accumulated sum of the positive nuclei present in the optical sections between 60 and 200μm below the top of collagen gels was expressed as a percentage of the total number of positive nuclei on top and within the collagen gel. This value, therefore, represents the proportion of total cells within each sample invading beyond a depth of 60μm and up to 200μm. |
||
| Animal Protocol |
|
||
| ADME/Pharmacokinetics |
Saracatinib reduces MD-MSC viability and has good nerve penetration [2]
A pharmacokinetic study of saracatinib (25 mg/kg oral dose) in NSG mice revealed that plasma and nerve concentrations of saracatinib peaked at 0.5 hours with a t1/2 of 4.4 hours, but remained constant in the nerve at approximately 300–400 ng/g for at least 8 hours, the longest time point measured (Fig. 3D). Saracatinib selectively reduced MD-MSC viability (IG50 = 0.3 μmol/L) but had a low maximum effect of approximately 40%–50%, compared with an 80% maximum effect for dasatinib (Fig. 3E). Saracatinib decreased the levels of Src(Y416) phosphorylation compared with total Src levels, and also decreased Src-dependent phosphorylation of FAK(Y576), and Src/FAK-dependent phosphorylation of paxillin(Y118) at 6 hours of treatment (Fig. 3F). After 24 hours of saracatinib treatment, MD-MSCs had increased p27 levels compared with controls (Fig. 3G). Pharmacokinetics [1] The AZD0530 plasma concentration 6h after oral dosing increased proportionally to the dose (Figure 4A). |
||
| References |
|
||
| Additional Infomation |
Saracatinib is a member of the class of quinazolines that is quinazoline substituted by (5-chloro-2H-1,3-benzodioxol-4-yl)amino, (oxan-4-yl)oxy and 2-(4-methylpiperazin-1-yl)ethoxy groups at positions 4, 5 and 7, respectively. It is a dual inhibitor of the tyrosine kinases c-Src and Abl (IC50 = 2.7 and 30 nM, respectively). Saracatinib was originally developed by AstraZeneca for the treatment of cancer but in 2019 it was granted orphan drug designation by the US Food and Drug Administration for the treatment of idiopathic pulmonary fibrosis (IPF), a type of lung disease that results in scarring (fibrosis) of the lungs. It has a role as an antineoplastic agent, an EC 2.7.10.2 (non-specific protein-tyrosine kinase) inhibitor, a radiosensitizing agent, an autophagy inducer, an apoptosis inducer and an anticoronaviral agent. It is a member of quinazolines, a secondary amino compound, a N-methylpiperazine, an aromatic ether, a member of oxanes, a member of benzodioxoles, an organochlorine compound and a diether.
Saracatinib has been investigated for the treatment of Cancer, Osteosarcoma, Ovarian Cancer, Fallopian Tube Cancer, and Primary Peritoneal Cancer. Saracatinib is an orally available 5-, 7-substituted anilinoquinazoline with anti-invasive and anti-tumor activities. Saracatinib is a dual-specific inhibitor of Src and Abl, protein tyrosine kinases that are overexpressed in chronic myeloid leukemia cells. This agent binds to and inhibits these tyrosine kinases and affects cell motility, cell migration, adhesion, invasion, proliferation, differentiation, and survival. Specifically, Saracatinib inhibits Src kinase-mediated osteoclast bone resorption. AZD0530, an orally available Src inhibitor, demonstrated potent antimigratory and anti-invasive effects in vitro, and inhibited metastasis in a murine model of bladder cancer. Antiproliferative activity of AZD0530 in vitro varied between cell lines (IC(50) 0.2 ->10μM). AZD0530 inhibited tumor growth in 4/10 xenograft models tested and dynamically inhibited in vivo phosphorylation of Src substrates paxillin and FAK in both growth-inhibition-resistant and -sensitive xenografts. The activity of AZD0530 in NBT-II bladder cancer cells in vitro was consistent with inhibition of cell migration and stabilization of cell-cell adhesion. These data suggest a dominant anti-invasive pharmacology for AZD0530 that may limit tumor progression in a range of cancers. AZD0530 is currently in Phase II clinical trials.[1] The data presented here support a mechanistic role of endogenous Src in promoting an invasive cell phenotype through the regulation of cell adhesion, migration, and invasion. AZD0530 inhibited cancer cell proliferation in vitro and in vivo in a constitutively active Src fibroblast tumor model, and in some human tumor cell lines. The lack of consistent antiproliferative activity and the underlying mechanism of such diverse responses require further investigation in additional tumor models. Effects on models of invasion were more clear cut: AZD0530 inhibited invasion and migration of cancer cells in vitro, and inhibited phosphorylation of proteins controlling migration in vitro and in vivo. In an in vivo model of bladder cancer metastasis, AZD0530 inhibited the number of lymph node metastases, detected through colony forming assays, thus recapitulating the effects seen with dominant‐negative Src and Csk constructs in earlier studies in this model (Boyer et al., 2002). We and others have recently demonstrated activity of AZD0530 in inhibiting metastasis in pancreatic cancer models (Green et al., 2005) and in an orthotopic model of human colorectal carcinoma (Phillips et al., 2007). Together with the results presented here, these data suggest that AZD0530 may provide clinical benefit by preventing or delaying tumor progression through inhibition of tumor cell migration and invasion. AZD0530 is currently in Phase II clinical trials.[1] Neurofibromatosis type 2 (NF2) is a nervous system tumor disorder caused by inactivation of the merlin tumor suppressor encoded by the NF2 gene. Bilateral vestibular schwannomas are a diagnostic hallmark of NF2. Mainstream treatment options for NF2-associated tumors have been limited to surgery and radiotherapy; however, off-label uses of targeted molecular therapies are becoming increasingly common. Here, we investigated drugs targeting two kinases activated in NF2-associated schwannomas, c-Met and Src. We demonstrated that merlin-deficient mouse Schwann cells (MD-MSC) treated with the c-Met inhibitor, cabozantinib, or the Src kinase inhibitors, dasatinib and saracatinib, underwent a G1 cell-cycle arrest. However, when MD-MSCs were treated with a combination of cabozantinib and saracatinib, they exhibited caspase-dependent apoptosis. The combination therapy also significantly reduced growth of MD-MSCs in an orthotopic allograft mouse model by greater than 80% of vehicle. Moreover, human vestibular schwannoma cells with NF2 mutations had a 40% decrease in cell viability when treated with cabozantinib and saracatinib together compared with the vehicle control. This study demonstrates that simultaneous inhibition of c-Met and Src signaling in MD-MSCs triggers apoptosis and reveals vulnerable pathways that could be exploited to develop NF2 therapies. [2] This study demonstrates that simultaneous c-Met and Src inhibition induced apoptosis of merlin-deficient mouse Schwann cells in culture and in allografts, and reduced growth of primary VS cells with NF2 mutations. The drug combination also decreased the viability of two merlin-deficient human SC lines and a human benign meningioma line in vitro (Supplementary Fig. S4), confirming that the effects are not cell-line or species-dependent. Both ERK and Src-FAK kinases converge onto the PI3K/Akt pathway; inhibitors of which induce apoptosis in NF2-associated cells. Considering the severe toxicity associated with direct targeting of PI3K with Idelalisib, and until additional PI3K inhibitors are approved, simultaneous inhibition of c-Met-ERK and Src-FAK pathways with cabozantinib and dasatinib/saracatinib may promote schwannoma cell apoptosis with fewer adverse effects. This study provides preclinical data supporting further investigation of dual inhibition of c-Met and Src as a potential therapy for NF2-associated schwannomas.[2] |
| Molecular Formula |
C27H32CLN5O5
|
|---|---|
| Molecular Weight |
542.0265
|
| Exact Mass |
541.209
|
| Elemental Analysis |
C, 59.83; H, 5.95; Cl, 6.54; N, 12.92; O, 14.76
|
| CAS # |
379231-04-6
|
| Related CAS # |
Saracatinib difumarate;893428-72-3
|
| PubChem CID |
10302451
|
| Appearance |
White to light yellow solid powder
|
| Density |
1.3±0.1 g/cm3
|
| Boiling Point |
671.3±55.0 °C at 760 mmHg
|
| Flash Point |
359.8±31.5 °C
|
| Vapour Pressure |
0.0±2.1 mmHg at 25°C
|
| Index of Refraction |
1.641
|
| LogP |
2.74
|
| Hydrogen Bond Donor Count |
1
|
| Hydrogen Bond Acceptor Count |
10
|
| Rotatable Bond Count |
8
|
| Heavy Atom Count |
38
|
| Complexity |
743
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
ClC1C([H])=C([H])C2=C(C=1N([H])C1C3=C(C([H])=C(C([H])=C3OC3([H])C([H])([H])C([H])([H])OC([H])([H])C3([H])[H])OC([H])([H])C([H])([H])N3C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])C3([H])[H])N=C([H])N=1)OC([H])([H])O2
|
| InChi Key |
OUKYUETWWIPKQR-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C27H32ClN5O5/c1-32-6-8-33(9-7-32)10-13-35-19-14-21-24(23(15-19)38-18-4-11-34-12-5-18)27(30-16-29-21)31-25-20(28)2-3-22-26(25)37-17-36-22/h2-3,14-16,18H,4-13,17H2,1H3,(H,29,30,31)
|
| Chemical Name |
N-(5-Chloro-1,3-benzodioxol-4-yl)-7-[2-(4-methyl-1-piperazinyl)ethoxy]-5-[(tetrahydro-2H-pyran-4-yl)oxy]-4-quinazolinamine
|
| Synonyms |
AZD0530; Saracatinib; AZD-0530; Saracatinib; 379231-04-6; AZD0530; Saracatinib (AZD0530); N-(5-Chloro-1,3-benzodioxol-4-yl)-7-[2-(4-methyl-1-piperazinyl)ethoxy]-5-[(tetrahydro-2H-pyran-4-yl)oxy]-4-quinazolinamine; Saracatinib [USAN]; AZD 0530
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (4.61 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (4.61 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 2.5 mg/mL (4.61 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 4: 2% DMSO+30% PEG 300+ddH2O: 5 mg/mL |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8449 mL | 9.2246 mL | 18.4492 mL | |
| 5 mM | 0.3690 mL | 1.8449 mL | 3.6898 mL | |
| 10 mM | 0.1845 mL | 0.9225 mL | 1.8449 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04307953 | Recruiting | Drug: AZD0530 Difumarate Drug: Matching placebo |
Fibrodysplasia Ossificans Progressiva | Amsterdam UMC, location VUmc | August 5, 2020 | Phase 2 |
| NCT02116712 | Completed | Drug: Saracatinib | Pulmonary Lymphangioleiomyomatosis | Tony Eissa | August 2014 | Phase 1 |
| NCT02732587 | Completed Has Results | Drug: Saracatinib | Alcohol Drinking | Yale University | November 2015 | Phase 1 |
| NCT02737202 | Terminated | Drug: saracatinib | Pulmonary Lymphangioleiomyomatosis | Baylor College of Medicine | April 2016 | Phase 2 |
|
 |
 |
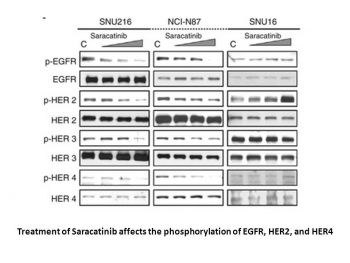
AZD0530 inhibits cell proliferation through β-catenin, ERK1/2 and GSK3β-mediated cyclin D1 and c-myc regulation.Oncogene.2008 Oct 23;27(49):6365-75. |
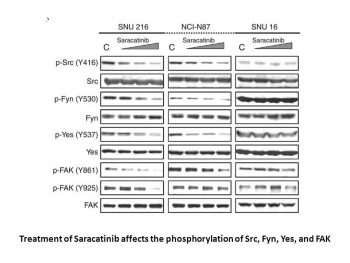
AZD0530 inhibits Src activation through inhibition of Y419 phosphorylation.Oncogene.2008 Oct 23;27(49):6365-75. |
AZD0530 inhibits cell migration through Src-mediated FAK activation.Oncogene.2008 Oct 23;27(49):6365-75. |