
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| Other Sizes |
Purity: ≥98%
| Targets |
PLK1 (IC50 = 9 nM); PDGFR (IC50 = 18 nM); Bcr-Abl (IC50 = 32 nM); Flt1 (IC50 = 42 nM); Src (IC50 = 155 nM)
|
|---|---|
| ln Vitro |
Rigosertib, which has an IC50 of 9 nM, is a non-ATP-competitive inhibitor of PLK1. Moreover, rigosertib shows inhibition with an IC50 of 18-260 nM against PLK2, PDGFR, Flt1, BCR-ABL, Fyn, Src, and CDK1. 94 distinct tumor cell lines, including BT27, MCF-7, DU145, PC3, U87, A549, H187, RF1, HCT15, SW480, and KB cells, exhibit cell killing activity in response to rigosertib, with an IC50 of 50–250 nM. Rigosertib, however, has little to no effect in normal cells, such as HFL, PrEC, HMEC, and HUVEC, unless the concentration is higher than 5–10 μM. Rigosertib (100-250 nM) causes spindle abnormalities and apoptosis in HeLa cells.[1] With an IC50 of 50-100 nM, rigorsertib also inhibits a number of multidrug-resistant tumor cell lines, such as MES-SA, MES-SA/DX5a, CEM, and CEM/C2a. Rigosertib (0.25–5 μM) causes an accumulation of cells with subG1 DNA content, initiates apoptotic pathways, and inhibits cell cycle progression in G2/M phase in DU145 cells. Rigosertib (50 nM-0.5 μM) causes caspase 3/7 activation and viability loss in A549 cells.[2] According to a recent study, rigosertib causes CLL (chronic lymphocytic leukemia) cells to undergo apoptosis without harming healthy B-cells or T-cells. Additionally, rigorsertib inhibits the migration of leukemic cells induced by SDF-1 and nullifies the pro-survival effect of follicular dendritic cells on CLL cells.[3]
|
| ln Vivo |
Rigosertib (250 mg/kg) significantly inhibits tumor growth in mouse xenograft models of MCF-7, MIA-PaCa, and Bel-7402 cells.[1] A mouse xengraft model of BT20 cells demonstrates the inhibitory effect of rigosertib (200 mg/kg) on tumor growth.[2]
|
| Enzyme Assay |
For 30 minutes at room temperature, recombinant PLK1 (10 ng) is incubated with varying concentrations of rigosertib in a 15 µL reaction mixture (50 mM HEPES, 10 mM MgCl2, 1 mM EDTA, 2 mM Dithiothreitol, 0.01% NP-40 [pH 7.5]). 20 µL (15 µL enzyme + inhibitor, 2 µL 1 mM ATP), 2 µL of γ32P-ATP (40 μCi), and 1 µL of recombinant Cdc25C (100 ng) or casein (1 μg) substrates are used in kinase reactions, which are carried out for 20 min at 30 °C. Boiling in 20 µL of 2× Laemmli buffer for 2 minutes ends the reaction. 18% SDS-PAGE is used to separate phosphorylated substrates. After drying, the gels are exposed to X-ray film for three to ten minutes.
|
| Cell Assay |
One day later, different concentrations of Rigosertib are added to six-well dishes containing 1×105 cells/mL/well of tumor cells that have been plated. After treatment for 96 hours, cell counts are obtained from duplicate wells. By using trypan blue exclusion, the total number of viable cells is found.
|
| Animal Protocol |
Mouse (female athymic, NCR-nu/nu) xenograft models of Bel-7402, MCF-7, and MIA-PaCa cells
250 mg/kg Intraperitonially |
| References | |
| Additional Infomation |
Rigosertib sodium is the sodium salt of rigosertib. It is an anti-cancer agent which has been granted Orphan Drug Designation by the FDA for use in patients with myelodysplastic syndromes (MDS). It has a role as a microtubule-destabilising agent, an antineoplastic agent, an EC 2.7.11.21 (polo kinase) inhibitor and an apoptosis inducer. It contains a rigosertib(1-).
Rigosertib Sodium is the sodium salt form of rigosertib, a synthetic benzyl styryl sulfone analogue and Ras mimetic, with potential antineoplastic activity. Upon administration, rigosertib targets and binds to Ras-binding domain (RBD) found in many Ras effector proteins, including Raf kinase and phosphatidylinositol 3-kinase (PI3K). This prevents Ras from binding to its targets and inhibits Ras-mediated signaling pathways, including Ras/Raf/Erk, Ras/CRAF/polo-like kinase1 (Plk1), and Ras/ PI3K/Akt signaling pathways. This induces cell cycle arrest and apoptosis and inhibits proliferation in a variety of susceptible tumor cells. See also: Rigosertib (annotation moved to). |
| Molecular Formula |
C21H24NNAO8S
|
|---|---|
| Molecular Weight |
473.47
|
| Exact Mass |
473.112
|
| Elemental Analysis |
C, 55.86; H, 5.58; N, 3.10; O, 28.35; S, 7.10
|
| CAS # |
1225497-78-8
|
| Related CAS # |
Rigosertib sodium;592542-60-4;Rigosertib;592542-59-1
|
| PubChem CID |
23696523
|
| Appearance |
white solid powder
|
| LogP |
2.622
|
| Hydrogen Bond Donor Count |
1
|
| Hydrogen Bond Acceptor Count |
9
|
| Rotatable Bond Count |
11
|
| Heavy Atom Count |
32
|
| Complexity |
684
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
S(/C(/[H])=C(\[H])/C1C(=C([H])C(=C([H])C=1OC([H])([H])[H])OC([H])([H])[H])OC([H])([H])[H])(C([H])([H])C1C([H])=C([H])C(=C(C=1[H])N([H])C([H])([H])C(=O)[O-])OC([H])([H])[H])(=O)=O.[Na+]
|
| InChi Key |
VLQLUZFVFXYXQE-USRGLUTNSA-M
|
| InChi Code |
InChI=1S/C21H25NO8S.Na/c1-27-15-10-19(29-3)16(20(11-15)30-4)7-8-31(25,26)13-14-5-6-18(28-2)17(9-14)22-12-21(23)24;/h5-11,22H,12-13H2,1-4H3,(H,23,24);/q;+1/p-1/b8-7+;
|
| Chemical Name |
sodium;2-[2-methoxy-5-[[(E)-2-(2,4,6-trimethoxyphenyl)ethenyl]sulfonylmethyl]anilino]acetate
|
| Synonyms |
ON-01910; Rigosertib sodium; ON01910; ON 01910; brand name: Estybon
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
|
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1121 mL | 10.5603 mL | 21.1207 mL | |
| 5 mM | 0.4224 mL | 2.1121 mL | 4.2241 mL | |
| 10 mM | 0.2112 mL | 1.0560 mL | 2.1121 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04177498 | Recruiting | Other: Quality-of-Life Assessment Drug: Rigosertib Sodium |
Recessive Dystrophic Epidermolysis Bullosa |
Thomas Jefferson University | August 24, 2021 | Early Phase 1 |
| NCT04263090 | Recruiting | Drug: Rigosertib Drug: Nivolumab |
Adenocarcinoma Stage IV |
Icahn School of Medicine at Mount Sinai |
June 29, 2020 | Phase 1 Phase 2 |
| NCT03786237 | Recruiting | Drug: Rigosertib Oral Capsules / Rigosertib Intravenous |
Epidermolysis Bullosa Dystrophica Squamous Cell Carcinoma |
Prof. Johann Bauer | April 12, 2021 | Phase 1 Phase 2 |
| NCT05764395 | Recruiting | Drug: Rigosertib Procedure: Biopsy |
Metastatic Melanoma Refractory Melanoma |
Vanderbilt-Ingram Cancer Center |
May 9, 2023 | Phase 2 |
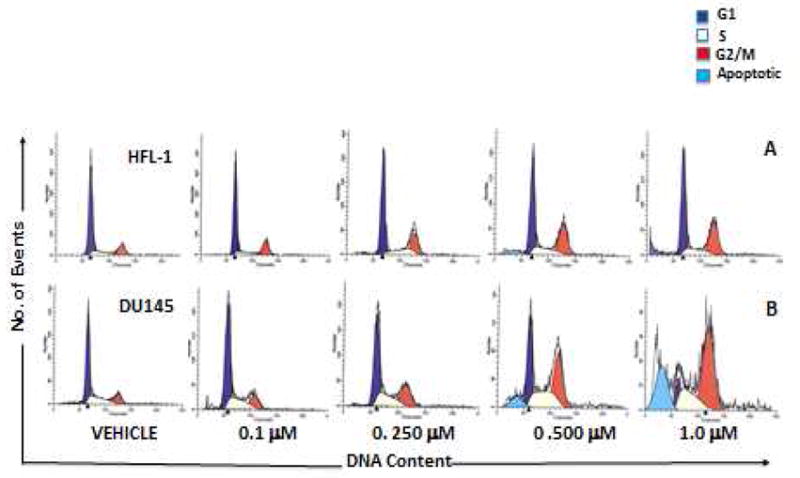 28(ON 01910.Na) selectively induces mitotic G2/M arrest and apoptosis in cancer cells.J Med Chem.2011 Sep 22;54(18):6254-76. |
|---|
DU145 and HFL-1 (normal human fibroblasts) cells were treated with increasing concentrations of28or DMSO (Vehicle) for 48 h.J Med Chem.2011 Sep 22;54(18):6254-76. |
Cellular viability together with the activity of caspases 3/7 were assayed concomitantly in A549 cells treated with28for 24 h (n=3).J Med Chem.2011 Sep 22;54(18):6254-76. |