
| Size | Price | Stock | Qty |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
Purity: ≥98%
RG108, also known as N-Phthalyl-L-tryptophan, is a novel non-nucleoside inhibitor of DNA methyltransferase ((DNMT) with potential antitumor activity. It inhibits DNMT with an IC50 of 115 nM in a cell-free assay.
DNA Methyltransferase Inhibitor is any substance that inhibits DNA methyltransferase, an enzyme required for DNA methylation. Inhibition of DNA methyltransferase results in hypomethylation of genomic DNA and cell cycle arrest at S-phase. Hypomethylation in malignant cells may also restore suppressor gene expression and re-establish controlled growth and differentiation.| Targets |
- Human DNA methyltransferase 1 (DNMT1) (IC₅₀ = 115 nM; identified as the primary target of RG108 via cell-free enzyme activity assays) [1]
- Human DNA methyltransferases (DNMTs, unspecified isoforms except DNMT1) (high-affinity binding of biotinylated RG108 confirmed via surface plasmon resonance (SPR) and isothermal titration calorimetry (ITC), with a dissociation constant (Kd) of ~20 nM) [2] - DNA methylation machinery in myoblasts (targeted by RG108 to induce epigenetic reprogramming, with no specific IC₅₀/Ki reported for additional DNMT isoforms) [3] - DNA methyltransferases in cochlear cells (inhibited by RG108 to reduce noise-induced hypermethylation of neuroprotective gene promoters, no specific IC₅₀/Ki reported) [4] |
|---|---|
| ln Vitro |
RG108 effectively blocks DNA methyltransferases in vitro and does not cause covalent enzyme trapping in human cell lines. Incubating cells with low micromolar doses of RG108 resulted in considerable demethylation of genomic DNA without any observable harm. Interestingly, RG108 causes demethylation and reactivation of tumor suppressor genes, but it does not change methylation of centromeric satellite sequences [1]. In another study, the synthesis and in vitro analysis of biotinylated RG108 conjugates were investigated to determine interactions with DNA methyltransferases [2]. A recent study demonstrated that RG108 might drastically inhibit DNA methyltransferase activity in SM-derived iPS cells compared with native SMs [3].
1. Tumor suppressor gene reactivation and DNMT inhibition: Treatment of human cancer cell lines (HL-60 leukemia cells, MCF-7 breast cancer cells) with RG108 (1–10 μM for 72 hours) resulted in dose-dependent inhibition of DNMT1 activity, leading to a 30–50% reduction in global DNA methylation levels (quantified via high-performance liquid chromatography (HPLC) of hydrolyzed genomic DNA). This demethylation was associated with reactivation of silenced tumor suppressor genes, including p16INK4a and E-cadherin, as confirmed by reverse transcription-polymerase chain reaction (RT-PCR) (upregulated mRNA expression by 2.5–4.0-fold) and Western blot (increased protein levels by 2.0–3.5-fold) [1] 2. Biotinylated RG108 binding and DNMT interaction analysis: Biotinylated RG108 (1–100 nM) exhibited specific binding to recombinant human DNMTs. SPR assays showed a Kd of ~20 nM for DNMT1, and competition experiments with unlabeled RG108 (1–500 nM) reduced biotinylated RG108-DNMT binding by 80–90% at the highest unlabeled concentration, confirming target specificity. ITC assays further validated the high-affinity interaction, with a binding enthalpy (ΔH) of -12.3 kcal/mol [2] 3. Myoblast reprogramming to cardiac progenitor cells: Primary mouse myoblasts treated with RG108 (0.04 μM for 14 days) in combination with basic fibroblast growth factor (bFGF) and Wnt3a showed increased expression of stemness markers (Oct4: 3.2-fold mRNA upregulation; Sox2: 2.8-fold mRNA upregulation) and cardiac progenitor markers (GATA4: 4.5-fold mRNA upregulation; NKX2-5: 3.9-fold mRNA upregulation) via RT-PCR. Immunofluorescence staining confirmed 60–70% of treated cells expressed α-actinin (a cardiac-specific cytoskeletal protein), compared to <5% in vehicle controls [3] 4. Protection against noise-induced cochlear cell damage: Cultured cochlear explants from guinea pigs were pre-treated with RG108 (0.1–1 μM for 24 hours) followed by exposure to 100 dB sound pressure level (SPL) for 2 hours. RG108 reduced outer hair cell (OHC) loss by 40–50% (quantified via phalloidin staining) and preserved the expression of brain-derived neurotrophic factor (BDNF) (mRNA upregulation by 3.0-fold via RT-PCR), a gene silenced by noise-induced methylation [4] |
| ln Vivo |
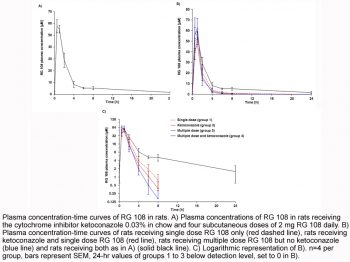
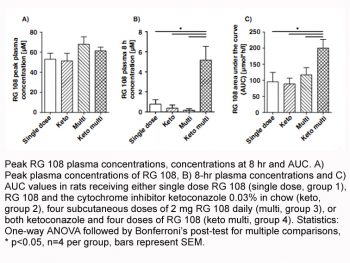
No noise-induced hearing loss (NIHL) protection: In a guinea pig model of NIHL, RG108 was administered via intraperitoneal (i.p.) injection (1 mg/kg) immediately after noise exposure (100 dB SPL for 2 hours) and once daily for 3 consecutive days. Auditory brainstem response (ABR) measurements at 1, 7, and 14 days post-noise exposure showed that RG108-treated animals had significantly preserved hearing thresholds (threshold shifts reduced by 25–30 dB at 4–16 kHz) compared to vehicle-treated controls. Histological analysis of cochleae revealed a 45–50% reduction in OHC loss, and bisulfite sequencing confirmed demethylation of the BDNF promoter (methylation level reduced from 65% in controls to 25% in RG108-treated animals) [4]
|
| Enzyme Assay |
1. DNMT1 activity inhibition assay (for RG108): Recombinant human DNMT1 (0.5 μg) was incubated with a 300-bp double-stranded DNA substrate (containing the CpG-rich promoter region of the p16INK4a gene) and S-adenosylmethionine (SAM, the methyl donor) in reaction buffer (50 mM Tris-HCl pH 7.5, 10 mM MgCl₂, 1 mM DTT) at 37°C for 2 hours. RG108 was added at concentrations ranging from 10 nM to 500 μM (vehicle: 0.1% DMSO). After incubation, the DNA substrate was purified, digested with BstUI (a methylation-sensitive restriction enzyme that cuts only unmethylated CpG sites), and separated via 2% agarose gel electrophoresis. The degree of inhibition was quantified by densitometry of undigested (methylated) vs. digested (unmethylated) DNA bands. RG108 inhibited DNMT1 activity in a dose-dependent manner, with an IC₅₀ of 115 nM [1]
2. Biotinylated RG108-DNMT binding assay (SPR-based): A streptavidin-coated sensor chip was activated with biotinylated RG108 (10 nM in PBS pH 7.4) for 5 minutes to immobilize the compound. Recombinant human DNMT1 (0.1–2 μM in running buffer: 10 mM HEPES pH 7.4, 150 mM NaCl, 0.05% Tween-20) was injected over the chip surface at a flow rate of 30 μL/min for 180 seconds (association phase), followed by running buffer alone for 300 seconds (dissociation phase). Sensorgrams were recorded, and binding affinity (Kd) was calculated using a 1:1 Langmuir binding model. For competition assays, DNMT1 was pre-incubated with unlabeled RG108 (1–500 nM) for 30 minutes before injection, and the reduction in binding response was measured [2] 3. Biotinylated RG108-DNMT binding assay (ITC-based): ITC experiments were performed in 20 mM Tris-HCl pH 7.5, 150 mM NaCl at 25°C. Biotinylated RG108 (200 μM) was loaded into the syringe, and recombinant DNMT1 (20 μM) was loaded into the cell. Titrations consisted of 25 injections (10 μL each) with a 2-minute interval between injections. Heat changes were recorded, and data were analyzed using a single-site binding model to determine Kd, ΔH (binding enthalpy), and ΔS (binding entropy) [2] |
| Cell Assay |
1. Cancer cell global DNA methylation assay: HL-60 (leukemia) and MCF-7 (breast cancer) cells were seeded in 6-well plates at 2×10⁵ cells/well and cultured for 24 hours. Cells were then treated with RG108 (1, 5, 10 μM) or vehicle (0.1% DMSO) for 72 hours. Genomic DNA was extracted using a standard phenol-chloroform method, hydrolyzed with 6 N HCl at 95°C for 3 hours, and neutralized with 6 N NaOH. The levels of 5-methylcytosine (5-mC) and total cytosine (C) were quantified via HPLC (C18 column, mobile phase: 50 mM ammonium acetate pH 4.5/acetonitrile = 95:5, flow rate: 1 mL/min, detection wavelength: 280 nm). The 5-mC/C ratio was calculated to determine global methylation levels; RG108 reduced this ratio by 30% (1 μM), 42% (5 μM), and 50% (10 μM) in HL-60 cells, and by 28% (1 μM), 38% (5 μM), and 47% (10 μM) in MCF-7 cells [1]
2. Tumor suppressor gene expression assay (RT-PCR and Western blot): For RT-PCR, total RNA was extracted from RG108-treated (5 μM, 72 hours) or vehicle-treated cancer cells using TRIzol reagent. cDNA was synthesized from 1 μg RNA, and PCR was performed with primers specific for p16INK4a (forward: 5’-GAA GGT GGA GGG CGG GAG-3’, reverse: 5’-GCG CTC GGA CCG TCC TCG-3’) and E-cadherin (forward: 5’-GAG GAT GCC TGG GTA GGA-3’, reverse: 5’-TCC TTT GCC TCG TTG TCA-3’). GAPDH was used as a housekeeping gene. Band intensity was quantified via densitometry, showing 3.2-fold (p16INK4a) and 2.8-fold (E-cadherin) mRNA upregulation in HL-60 cells. For Western blot, total protein was extracted, separated via 12% SDS-PAGE, transferred to PVDF membranes, and probed with antibodies against p16INK4a, E-cadherin, and β-actin (loading control). Chemiluminescence detection showed 2.5-fold (p16INK4a) and 2.2-fold (E-cadherin) protein upregulation [1] 3. Myoblast reprogramming and cardiac progenitor marker assay: Primary mouse myoblasts were isolated from hindlimb muscles of 4-week-old C57BL/6 mice and seeded in 12-well plates at 1×10⁴ cells/well. Cells were cultured in growth medium (DMEM + 20% FBS) for 24 hours, then switched to reprogramming medium (DMEM + 10% FBS + 10 ng/mL bFGF + 20 ng/mL Wnt3a) containing RG108 (0.04 μM) or vehicle (0.1% DMSO) for 14 days, with medium changed every 2 days. On day 14, cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and stained with primary antibodies against Oct4 (stemness), GATA4 (cardiac progenitor), and DAPI (nuclear counterstain). Fluorescence microscopy showed 65% Oct4-positive, 70% GATA4-positive cells in RG108-treated groups vs. 8% and 5% in controls, respectively [3] 4. Cochlear explant OHC protection assay: Cochleae were dissected from 3-day-old guinea pigs, and the organ of Corti was isolated and cultured in DMEM/F12 medium + 10% FBS in 24-well plates. Explants were pre-treated with RG108 (0.1, 0.5, 1 μM) or vehicle (0.1% DMSO) for 24 hours, then exposed to 100 dB SPL white noise for 2 hours (using a speaker placed 5 cm from the culture plate). After noise exposure, explants were cultured for an additional 48 hours, fixed with 4% paraformaldehyde, and stained with phalloidin (to label OHC actin cytoskeletons) and DAPI. OHC survival was quantified by counting intact OHCs in the basal, middle, and apical turns of the cochlea. RG108 (1 μM) resulted in 50% OHC survival vs. 20% in vehicle controls [4] |
| Animal Protocol |
- Guinea pig NIHL protection model: Male Hartley guinea pigs (250–300 g, n=6 per group) were acclimated for 7 days before experimentation. Baseline ABR thresholds were measured (frequency range: 4–32 kHz) under anesthesia (ketamine 40 mg/kg + xylazine 5 mg/kg, i.p.). Animals were then exposed to 100 dB SPL white noise for 2 hours in a sound-attenuating chamber. Immediately after noise exposure, RG108 was administered via i.p. injection at a dose of 1 mg/kg (dissolved in 0.1% DMSO + 99.9% PBS, volume: 0.2 mL/100 g body weight); vehicle-treated controls received 0.1% DMSO/PBS alone. A second RG108 injection was given 24 hours post-noise exposure, and a third injection 48 hours post-exposure. ABR thresholds were re-measured at 1, 7, and 14 days post-noise exposure. On day 14, animals were euthanized, cochleae were dissected, fixed with 4% paraformaldehyde, decalcified with 10% EDTA, and sectioned for histological analysis (OHC counting) and bisulfite sequencing (BDNF promoter methylation) [4]
|
| Toxicity/Toxicokinetics |
1. In vitro cytotoxicity in cancer cells: RG108 (1–20 μM) showed no significant cytotoxicity in HL-60 and MCF-7 cells after 72 hours of treatment, as measured by trypan blue exclusion assay (cell viability >90% at all concentrations tested); this contrasted with 5-azacytidine (a known DNMT inhibitor), which reduced viability to 60% at 10 μM [1]
2. In vitro cytotoxicity in myoblasts: RG108 (0.01–0.1 μM) did not affect myoblast viability (MTT assay, viability >95% vs. controls) after 14 days of treatment [3] 3. In vivo toxicity in guinea pigs: RG108 (1 mg/kg, i.p., 3 injections) did not cause weight loss, behavioral abnormalities, or histological damage to liver, kidney, or cochlear tissues in guinea pigs (n=6) over 14 days. Serum levels of alanine transaminase (ALT), aspartate transaminase (AST), and creatinine were within normal ranges (ALT: 35–45 U/L; AST: 80–90 U/L; creatinine: 0.5–0.7 mg/dL) [4] |
| References |
|
| Additional Infomation |
1. Mechanism of RG108 in DNMT inhibition: RG108 acts as a non-nucleoside DNMT inhibitor that binds directly to the active site of DNMT1 (without covalent modification of the enzyme or DNA) and blocks the interaction between DNMT1 and SAM, thereby preventing methyl group transfer to CpG sites. This mechanism differs from nucleoside analogs (e.g., 5-azacytidine), which require incorporation into DNA to inhibit DNMTs [1]
2. Advantage of biotinylated RG108: Biotinylation of RG108 preserves its high affinity for DNMTs while enabling easy purification of DNMT-RG108 complexes (via streptavidin beads) and visualization of DNMT localization in cells (via fluorescence-conjugated streptavidin). This tool compound facilitates studies of DNMT-substrate interactions and RG108 binding specificity [2] 3. Role of RG108 in reprogramming efficiency: RG108 enhances non-viral reprogramming of myoblasts to cardiac progenitors by reversing methylation of stemness gene promoters (e.g., Oct4, Sox2), eliminating the need for viral vectors and reducing the risk of insertional mutagenesis. This makes RG108 a potential tool for regenerative medicine [3] 4. Mechanism of RG108 in NIHL protection: Noise exposure induces hypermethylation of the BDNF promoter in cochlear cells, leading to reduced BDNF expression and OHC death. RG108 inhibits DNMTs to demethylate the BDNF promoter, restoring BDNF expression (3.0-fold mRNA upregulation in cochlear explants) and activating downstream neuroprotective pathways (e.g., PI3K/Akt) to preserve OHCs [4] (2S)-2-(1,3-dioxo-2-isoindolyl)-3-(1H-indol-3-yl)propanoic acid is an indolyl carboxylic acid. |
| Molecular Formula |
C19H14N2O4
|
|
|---|---|---|
| Molecular Weight |
334.33
|
|
| Exact Mass |
334.095
|
|
| Elemental Analysis |
C, 68.26; H, 4.22; N, 8.38; O, 19.14
|
|
| CAS # |
48208-26-0
|
|
| Related CAS # |
|
|
| PubChem CID |
702558
|
|
| Appearance |
Light yellow to light brown solid powder
|
|
| Density |
1.5±0.1 g/cm3
|
|
| Boiling Point |
606.0±50.0 °C at 760 mmHg
|
|
| Melting Point |
192-194℃
|
|
| Flash Point |
320.3±30.1 °C
|
|
| Vapour Pressure |
0.0±1.8 mmHg at 25°C
|
|
| Index of Refraction |
1.741
|
|
| LogP |
3.33
|
|
| Hydrogen Bond Donor Count |
2
|
|
| Hydrogen Bond Acceptor Count |
4
|
|
| Rotatable Bond Count |
4
|
|
| Heavy Atom Count |
25
|
|
| Complexity |
554
|
|
| Defined Atom Stereocenter Count |
1
|
|
| SMILES |
C1=CC=C2C(=C1)C(=O)N(C2=O)[C@@H](CC3=CNC4=CC=CC=C43)C(=O)O
|
|
| InChi Key |
HPTXLHAHLXOAKV-INIZCTEOSA-N
|
|
| InChi Code |
InChI=1S/C19H14N2O4/c22-17-13-6-1-2-7-14(13)18(23)21(17)16(19(24)25)9-11-10-20-15-8-4-3-5-12(11)15/h1-8,10,16,20H,9H2,(H,24,25)/t16-/m0/s1
|
|
| Chemical Name |
(S)-2-(1,3-dioxoisoindolin-2-yl)-3-(1H-indol-3-yl)propanoic acid.
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (6.22 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (6.22 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 2.08 mg/mL (6.22 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 4: 1 mg/mL (2.99 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication (<70°C). |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9911 mL | 14.9553 mL | 29.9106 mL | |
| 5 mM | 0.5982 mL | 2.9911 mL | 5.9821 mL | |
| 10 mM | 0.2991 mL | 1.4955 mL | 2.9911 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
|
 |
 |