
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g | |||
| Other Sizes |
Purity: ≥98%
Quizartinib (formerly also know as AC-220; AC-010220, brand name Vanflyta in Japan; Vanflyta) is a novel, potent, 2nd-generation, and orally bioavailable FLT3 tyrosine kinase inhibitor for Flt3 (ITD/WT) with potential anticancer activity.With IC50s of 1.1 nM and 4.2 nM, respectively, it inhibits FLT3 in MV4-11 and RS4EL11 cells. With respect to KIT, PDGFRα, PDGFRβ, RET, and CSF-1R, it demonstrates a ten-fold greater selectivity. Currently, Daiichi Sankyo is developing quizartinib to treat acute myeloid leukemia. Quizartinib (Vanflyta) was approved in 2023 by FDA for treating AML.
| Targets |
Flt3 (Kd = 1.6±0.7 nM)
|
|---|---|
| ln Vitro |
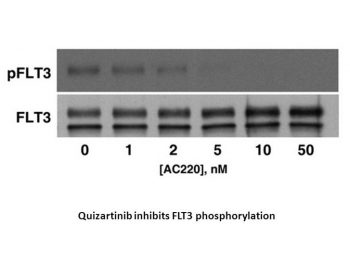
Quizartinib (AC220) is a novel substance designed specifically to inhibit FLT3 in the treatment of acute myeloid leukemia (AML). With an IC50 of 4.2±0.3 nM for FLT3-WT and 1.1±0.1 nM for FLT3-ITD, respectively, quizartinib inhibits FLT3-autophosphorylation. With an IC50 of 0.56±0.3 nM and >10,000 nM, respectively, quizartinib inhibits MV4-11 and A375 cells. When screened against most of the human protein kinome, quizartinib is highly selective and inhibits FLT3 with low nanomolar potency in cellular assays[1].
|
| ln Vivo |
Quizartinib (AC220) obliterates tumors in a FLT3-dependent mouse xenograft model at 10 mg/kg, potently inhibits FLT3 activity in primary patient cells, and significantly prolongs survival in a mouse model of FLT3-ITD AML at doses as low as 1 mg/kg when administered orally once daily. When comparing the oral and intravenous pharmacokinetics of quizartinib at 3 mg/kg in rats, the oral bioavailability was found to be roughly 40%. Mice are given a single oral gavage dose of quizartinib at a rate of 10 mg/kg, and they are killed twice after the dose in groups of four animals each. Time-dependent inhibition of FLT3 autophosphorylation was found in tumor samples when total FLT3 and phospho-FLT3 were quantified. After administration, FLT3 activity is reduced by 90% after two hours and 40% after twenty-four hours. Therefore, based on pharmacokinetic experiments, the degree of inhibition correlated favorably with the anticipated free Quizartinib plasma levels[1].
|
| Enzyme Assay |
Kinase binding experiments using KinomeScan are conducted. The kinase construct used in the FLT3 assay spanned only the catalytic domain (amino acids 592 to 969). The juxtamembrane domain is absent from this construct, which is intended to quantify the intrinsic binding affinity of inhibitors to the open FLT3 active site[1].
|
| Cell Assay |
The cells MV4-11 and RS4;11 are cultivated in Iscove media supplemented with 10% fetal bovine serum (FBS) and RPMI complete with 10% FBS, respectively. In order to perform proliferation assays, cells are seeded at a density of 40,000 cells per well in a 96-well plate after being cultured for an entire night in low serum media (0.5% FBS). The cells are supplemented with inhibitors (such as quizartinib) and incubated for 72 hours at 37°C. The Cell Titer-Blue Cell Viability Assay is used to measure cell viability. Cells are cultured in low serum medium (0.5% FBS) overnight, and the next day, they are seeded at a density of 400 000 cells per well in a 96-well plate to measure the inhibition of FLT3 autophosphorylation. Inhibitors, such as quizartinib, are cultured in the cells for two hours at 37°C. The 2-hour compound incubation is followed by a 15-minute addition of 100 ng/mL FLT3 ligand to cause FLT3 autophosphorylation in RS4;11 cells. Prepared cell lysates are incubated in 96-well plates that have been coated with a total FLT3 capture antibody beforehand. Either a biotinylated FLT3 antibody or an anti-phosphotyrosine antibody is used to incubate on the coated plates in order to detect total FLT3 or FLT3 autophosphorylation. For electrochemiluminescence detection on the Meso Scale Discovery platform, a SULFO-tagged streptavidin secondary antibody is utilized in both situations[1].
|
| Animal Protocol |
Mice: The mice used are female nu/NU or severe combined immunodeficient mice. Quizartinib (hydrochloride salt) is formulated in 22% hydroxypropyl-β-cyclodextrin, CEP-701 is formulated in 20% gelucire 44/14 in water (vol/vol), MLN-518 and SU 11248 are formulated in 10 mM sodium citrate (pH 3.5), PKC-412 is formulated in 3:1 gelucire 44/14-propylene glycol (vol/vol), and Bay 43-9006 is formulated in 80% PEG-400. Compound concentrations are selected in a volume of 10 mL/kg to deliver the intended dose. Oral gavage is used to administer compounds, and plasma samples are taken 0,25,0.5,1,2,4,6, and 24 hours after dosing. In order to obtain three independent plasma concentration time courses, eye bleeds (150 μL) are obtained semilongitudinally using three groups of three animals each, taking two to three time points per animal. Using four volumes of acetonitrile containing an internal standard, plasma samples and controls (25 μL) are extracted, and liquid chromatography tandem mass spectrometry is used for analysis.
Pharmacokinetic studies[1] Female NU/NU or severe combined immunodeficient mice were purchased from Charles River Laboratories or Harlan. AC220 (hydrochloride salt) was formulated in 22% hydroxypropyl-β-cyclodextrin, CEP-701 was formulated in 20% gelucire 44/14 in water (vol/vol), MLN-518 and sunitinib were formulated in 10 mM sodium citrate (pH 3.5), PKC-412 was formulated in 3:1 gelucire 44/14–propylene glycol (vol/vol), and sorafenib (toluene sulfonate salt) was formulated in 80% PEG-400. Compound concentrations were chosen to deliver the desired dose in a volume of 10 mL/kg. Compounds were administered by oral gavage and plasma samples collected 0.25, 0.5, 1, 2, 4, 6, and 24 hours after dosing. To collect plasma samples, eye bleeds (150 μL) were taken semilongitudinally using 3 groups of 3 animals each, taking 2 to 3 time points per animal to obtain a total of 3 independent plasma concentration time courses. Plasma samples and controls (25 μL) were extracted with 4 volumes of acetonitrile containing an internal standard and analyzed by liquid chromatography tandem mass spectrometry. Pharmacokinetic parameters were obtained by fitting the normalized liquid chromatography tandem mass spectrometry peak areas to a noncompartmental model using the linear trapezoidal estimation method in the WinNonlin software package. Mouse studies at Ambit complied with the recommendations of the “Guide for Care and Use of Laboratory Animals”45 with respect to restraint, husbandry, surgical procedures, feed and fluid regulation, and veterinary care. Animal efficacy studies[1] Subcutaneous xenograft model.[1] This model was performed at Ambit to measure in vivo inhibition of FLT3, and by Piedmont Research Center LLC to determine antitumor efficacy, following published procedures. Compounds were formulated and administered as described for pharmacokinetic studies. To measure FLT3 inhibition, tumors were harvested at 2 or 24 hours after compound administration, weighed, and lysed by mechanical dissociation. Tumor lysates were cleared of protein and tissue fragments by centrifugation at 835g for 15 minutes. Cleared lysates were assayed for total and phosphorylated FLT3 using the electrochemiluminescence-based enzyme-linked immunoassay (ELISA) described in “Cellular assays.” Bone marrow engraftment model.[1] The model was performed according to published procedures.20 For intravenous bone marrow engraftment, nonobese diabetic/severe combined immunodeficient mice were acclimated for 2 weeks before pretreatment with 150 mg/kg cyclophosphamide delivered intraperitoneally once a day for 2 days. After a 48-hour rest period, animals were given an intravenous injection of 5 × 106 MV4-11 cells into the tail vein. AC220 was formulated and delivered as described for pharmacokinetic studies. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
The mean (SD) absolute bioavailability of quizartinib from the tablet formulation was 71% (±7%) in healthy subjects. After oral administration under fasted conditions, time to peak concentration (median Tmax) of quizartinib and AC886 measured post dose was approximately 4 hours (range 2 to 8 hours) and 5 to 6 hours (range 4 to 120 hours), respectively, in healthy subjects. Following the administration of 35.4 mg quizartinib once daily in patients with newly diagnosed acute myeloid leukemia, the Cmax and AUC0-24h were calculated to be 140 ng/mL (71%) and 2,680 ng.h/mL (85%) respectively during the induction therapy and 204 ng/mL (64%) and 3,930 ng.h/mL (78%) respectively during the consolidation therapy. For the metabolite AC886, the Cmax and AUC0-24h were estimated to be 163 ng/mL (52%) and 3,590 ng.h/mL (51%) respectively during the induction therapy and 172 ng/mL (47%) and 3,800 ng.h/mL (46%) respectively during the consolidation therapy. Increasing the once daily dose of quizartinib to 53 mg also increases the Cmax and AUC0-24h of quizartinib to 529 ng/mL (60%) and 10,200 ng.h/mL (75%) respectively at steady state. The Cmax and AUC0-24h of the metabolite AC886 also increases to 262 ng/mL (48%) and 5,790 ng•h/mL (46%) respectively. No clinically significant differences in the pharmacokinetics of quizartinib were observed when administered with a high-fat, high-calorie meal. Following a single radiolabeled dose of quizartinib 53 mg to healthy subjects, 76.3% of the total radioactivity was recovered in feces (4% unchanged) and 1.64% in urine. Volume of distribution at steady state in healthy subjects was estimated to be 275 L (17%). Total body clearance of quizartinib in healthy subjects was estimated to be 2.23 L/hour (29%). Metabolism / Metabolites In vitro quizartinib is primarily metabolized via oxidation by CYP3A4/5 and AC886 is formed and metabolized by CYP3A4/5. Biological Half-Life The mean (SD) effective half-lives (t1/2) in patients with newly diagnosed AML for quizartinib and AC886 during maintenance therapy are 81 hours (±73) and 136 hours (±113), respectively. |
| Toxicity/Toxicokinetics |
Hepatotoxicity
In the prelicensure clinical trials of quizartinib in patients with AML, ALT elevations were arose in 10% to 16% of patients and were above 5 times the upper limit of normal (ULN) in 1% to 3%. However, similar rates were reported in subjects receiving chemotherapy without quizartinib and in most instances the elevations were transient, asymptomatic, and not associated with elevations in serum bilirubin. Intermittent elevations in liver enzymes are not uncommon in patients with untreated AML due to bacterial, viral and opportunistic infections. In the registration trials of quizartinib there were uncommon instances of acute liver injury and hepatic failure, but all were attributable to other comorbidities and factors (multiorgan failure), and none were considered due to quizartinib. Since its approval in the United States, there have been no reported cases of clinically apparent liver injury associated with quizartinib therapy. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of quizartinib during breastfeeding. Because quizartinib is more than 99% bound to plasma proteins, the amount in milk is likely to be low. However, the manufacturer recommends that breastfeeding be discontinued during quizartinib therapy and for 1 month after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding In vitro plasma protein binding of quizartinib and AC886 is 99% or greater. In vitro blood-to-plasma ratio for quizartinib and AC886 ranges from 0.79-1.30 and 1.36-3.19, respectively. |
| References |
|
| Additional Infomation |
Pharmacodynamics
Quizartinib showed antitumor activity in a mouse model of FLT3-ITD-dependent leukemia. In vitro, studies have shown that quizartinib is a predominant inhibitor of the slow delayed rectifier potassium current, IKs. In AML patients receiving quizartinib at a dose of 90 mg/day for females and 135 mg/day for males on a 28-day schedule, the median levels of phospho-FLT3 (pFLT3) and total FLT3 (tFLT3) decreased from 3312 RLU or 5639 RLU respectively at day 1 to 1235 RLU and 142 RLU respectively at day 8. Additionally, pFLT3 levels are statistically significantly higher (p < 0.0001, Mann Whitney test) for the ITD+ subjects on day 1; however, pFLT3 levels was reduced to a similar level in patients with or without the ITD mutation. The exposure-response analysis predicted a concentration-dependent QTcF interval median prolongation of 18 and 24 ms [upper bound of 2-sided 90% confidence interval (CI): 21 and 27 ms] at the median steady-state Cmax of quizartinib at the 26.5 mg and 53 mg dose level during maintenance therapy. |
| Molecular Formula |
C29H32N6O4S
|
|---|---|
| Molecular Weight |
560.67
|
| Exact Mass |
560.22
|
| Elemental Analysis |
C, 62.13; H, 5.75; N, 14.99; O, 11.41; S, 5.72
|
| CAS # |
950769-58-1
|
| Related CAS # |
1132827-21-4 (HCl);950769-58-1;
|
| PubChem CID |
24889392
|
| Appearance |
White to light yellow solid powder
|
| Density |
1.4±0.1 g/cm3
|
| Index of Refraction |
1.691
|
| LogP |
4.03
|
| Hydrogen Bond Donor Count |
2
|
| Hydrogen Bond Acceptor Count |
8
|
| Rotatable Bond Count |
8
|
| Heavy Atom Count |
40
|
| Complexity |
849
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
O=C(NC1C=CC(C2=CN3C(SC4C3=CC=C(OCCN3CCOCC3)C=4)=N2)=CC=1)NC1C=C(C(C)(C)C)ON=1
|
| InChi Key |
CVWXJKQAOSCOAB-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C29H32N6O4S/c1-29(2,3)25-17-26(33-39-25)32-27(36)30-20-6-4-19(5-7-20)22-18-35-23-9-8-21(16-24(23)40-28(35)31-22)38-15-12-34-10-13-37-14-11-34/h4-9,16-18H,10-15H2,1-3H3,(H2,30,32,33,36)
|
| Chemical Name |
1-(5-tert-butyl-1,2-oxazol-3-yl)-3-[4-[6-(2-morpholin-4-ylethoxy)imidazo[2,1-b][1,3]benzothiazol-2-yl]phenyl]urea
|
| Synonyms |
Quizartinib; AC220 or AC010220; AC 220; Quizartinib; 950769-58-1; AC220; Quizartinib (AC220); 1-(5-(tert-butyl)isoxazol-3-yl)-3-(4-(7-(2-morpholinoethoxy)benzo[d]imidazo[2,1-b]thiazol-2-yl)phenyl)urea; Quizartinib HCl; AC-220; AC-010220; AC 010220;Vanflyta
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 1 mg/mL (1.78 mM) (saturation unknown) in 10% DMF 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 1 mg/mL (1.78 mM) (saturation unknown) in 10% DMF 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: 1 mg/mL (1.78 mM) in 10% DMF 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Solubility in Formulation 4: 15% Captisol: 30mg/mL |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.7836 mL | 8.9179 mL | 17.8358 mL | |
| 5 mM | 0.3567 mL | 1.7836 mL | 3.5672 mL | |
| 10 mM | 0.1784 mL | 0.8918 mL | 1.7836 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02668653 | Active Recruiting |
Drug: Quizartinib Drug: Placebo |
Leukemia Acute Myeloid Leukemia |
Daiichi Sankyo, Inc. | September 2016 | Phase 3 |
| NCT04107727 | Active Recruiting |
Drug: Quizartinib Drug: Cytarabine |
Acute Myeloid Leukemia | PETHEMA Foundation | September 5, 2019 | Phase 2 |
| NCT04493138 | Recruiting | Drug: Quizartinib Drug: Azacitidine |
Myelodysplastic Syndrome Myeloproliferative Neoplasm |
M.D. Anderson Cancer Center | July 21, 2020 | Phase 1 Phase 2 |
| NCT04128748 | Recruiting | Drug: Quizartinib Drug: Liposome-encapsulated Daunorubicin-Cytarabine |
Acute Myeloid Leukemia High Risk Myelodysplastic Syndrome |
M.D. Anderson Cancer Center | May 27, 2020 | Phase 1 Phase 2 |
|
 |