
| Size | Price | Stock | Qty |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| Other Sizes |
|
Purity: ≥98%
Rilzabrutinib (PRN-1008) is a novel, highly potent, and reversible covalent inhibitor of BTK (Bruton's Tyrosine Kinase) being studied for treating rheumatoid arthritis. It inhibits BTK with an IC50 of 1.3 nM. BTK is a Tec family tyrosine kinase, is critical in immune pathways as an essential intracellular signaling element, participating in both adaptive and immune responses. PRN1008 was found to be very potent against BTK and highly selective when tested against a panel of 251 other kinases. Cysteine targeting of BTK by PRN1008 results in a slow off-rate demonstrated by retention of 79 ± 2% of binding to BTK in PBMC 18 hours after washing away the compound in vitro. PRN1008 was safe and well-tolerated following oral administration, and achieved high, sustained levels of BTK occupancy in peripheral blood mononuclear cells. PRN1008 is currently under Phase I development as a therapeutic agent for rheumatoid arthritis.
| Targets |
BTK (IC50 = 1.3 nM); BMX (IC50 = 1.0 nM); ITK (IC50 = 440 nM); TEC (IC50 = 0.8 nM); RLK (IC50 = 1.2 nM); BLK (IC50 = 6.3 nM); EGFR (IC50 = 520 nM); ERBB2 (IC50 = 3900 nM); ERBB4 (IC50 = 11.3 nM)
|
||
|---|---|---|---|
| ln Vitro |
With an IC50 of 1.3±0.5 nM, rilzabrutinib is a reversible covalent inhibitor of Bruton's tyrosine kinase (BTK). Additionally, when rilzabrutinib was evaluated against a panel of 251 additional kinases, it was shown to be extremely selective. Rilzabrutinib, which targets the cysteine of BTK, caused a delayed dissociation rate; 18 hours after the chemical was washed away in vitro, 79±2% of bound BTK was still present in PBMC. Complete reversibility of covalent cysteine binding occurs upon target denaturation. Rilzabrutinib suppressed human B cell proliferation (10% serum) produced by anti-IgM and B cell CD69 expression, with IC50 values of 5±2.4 nM and 123±38 nM, respectively [2].
|
||
| ln Vivo |
Following the drug's removal from the bloodstream, rilzabrutinib continues to have pharmacodynamic effects that are consistent with a prolonged target residence duration. Additionally, in a dose-dependent manner, rilzabrutinib reversed and totally suppressed collagen-induced arthritis in rats, which associated target occupancy with the alleviation of the disease [2].
|
||
| Animal Protocol |
|
||
| ADME/Pharmacokinetics |
Cmax was highly correlated with the magnitude of BTK occupancy 4 h post‐PRN1008 dosing (Figure 4). A sigmoidal Emax model, with a fitted γ and E0 provided the best fit of PRN1008 Cmax vs. occupancy data. The parameter estimates (% coefficient of variation) were: Emax 76.7 (13) %; EC50 46.5 (15) ng ml–1; E0 17.8 (42) %, and γ 2.1 (31). These results suggest a fairly steep exposure–response relationship, with low variability, and with 80% of maximal occupancy achieved at Cmax concentrations of approximately 100 ng ml–1 and above. The E0 value of 17.8% is consistent with the lower quantifiable range of the assay.
The robust relationship between PRN1008 Cmax and 4‐h occupancy, along with the very consistent occupancy decay rate of ~1.6% h–1 should allow for design of dosing regimens (dose and dose intervals) which precisely target various levels of occupancy over the course of a dose interval. It is noted that the nature of the PK and PK/PD relationships of PRN1008 may differ between healthy volunteers and patient populations, and should be further evaluated in future studies in patients.[1] This single-center, open-label, non-randomized, two-part, phase I study was conducted (1) to evaluate the absolute oral bioavailability of rilzabrutinib 400 mg tablet following an i.v. microtracer dose of ~100 μg [14C]-rilzabrutinib (~1 μCi) and single oral dose of 400 mg rilzabrutinib tablet (part 1), and (2) to characterize the absorption, metabolism, and excretion (AME) of 14C-radiolabeled rilzabrutinib following single oral dose (300 mg) of [14C]-rilzabrutinib (~1000 μCi; administered as a liquid) in healthy male participants (part 2). A total of 18 subjects were enrolled (n = 8 in part 1; n = 10 in part 2). The absolute bioavailability of 400 mg rilzabrutinib oral tablet was low (<5%). In part 1, rilzabrutinib was absorbed rapidly after single oral dose of rilzabrutinib 400 mg tablet with a median (range) time to maximum concentration (Tmax ) value of 2.03 h (1.83-2.50 h). The geometric mean (coefficient of variation) terminal half-life following the oral dose and i.v. microtracer dose of ~100 μg [14C]-rilzabrutinib, were 3.20 (51.0%) and 1.78 (37.6%) h, respectively. In part 2, rilzabrutinib was also absorbed rapidly following single oral dose of 300 mg [14C]-rilzabrutinib solution with a median (range) Tmax value of 1.00 h (1.00-2.00 h). The majority of total radioactivity was in the feces for both non-bile collection subjects (92.9%) and bile collection subjects (87.6%), and ~5% of radioactivity was recovered in urine after oral administration. Urinary excretion of unchanged rilzabrutinib was low (3.02%). The results of this study advance the understanding of the absolute bioavailability and AME of rilzabrutinib and can help inform its further investigation.Clin Transl Sci. 2023 Jul;16(7):1210-1219. |
||
| Toxicity/Toxicokinetics |
Data from all 80 enrolled subjects who received study drug (either PRN1008 or placebo) were included in the safety population. All participants were assessed for AEs for the duration of the study. No serious AEs or deaths were reported during the study, and no participants discontinued treatment due to an AE in either Part A or Part B.
At PRN1008 single doses of 50 to 600 mg, safety and tolerability was similar to placebo. In these four cohorts, there was only one subject of the six treated in each cohort who experienced a treatment‐emergent AE (TEAE). Of these four TEAEs, only one was considered related to study drug (nausea in Cohort A4), and one was graded as moderate (toothache in Cohort A2, unrelated to study drug). This compares with two TEAEs reported in two of the 10 subjects who received placebo (both graded as mild, not drug related).
In Cohort A5 (1200 mg), the primary AEs observed were gastrointestinal (GI) in nature, and were reported by each of the six subjects receiving PRN1008. The drug‐related AEs included diarrhoea (coded as loose stools; n = 6, 3 mild and 3 moderate severity), nausea (n = 3, 2 mild and 1 moderate severity), vomiting (n = 1), throat irritation (n = 3), and oropharyngeal discomfort (n = 1).
There was no apparent relationship between GI AEs and PRN1008 pharmacokinetics. As described above, both Cmax and area under the concentration–time curve for PRN1008 were similar at the 600 mg and 1200 mg doses. Despite similar plasma PK, the increase in GI AEs for the 600 mg vs. 1200 mg dose levels would suggest a localized effect related to total administered dose, and not to plasma exposure.
Following 10 or 11 days of dosing, PRN1008 was generally safe and well tolerated. TEAEs were reported in 7/8, 4/8, 8/8, 7/8 and 4/8 subjects in the 300 mg QD, 300 mg twice daily (BID), 600 mg QD, 450 mg BID and placebo groups, respectively. TEAEs classified as treatment‐related appeared to be more frequent in PRN1008 receiving subjects, reported in 6/8, 3/8, 8/8, 6/8 and 1/8 subjects in the 300 mg QD, 300 mg BID, 600 mg QD, 450 mg BID and placebo groups, respectively. All TEAEs classified as related to study drug were mild in intensity, with the exception of one subject in the 450 mg BID cohort who reported moderate diarrhoea.
There were no clinically significant or dose‐dependent changes observed in haematology, biochemistry or coagulation laboratory parameters in either Part A or Part B of the study. Similarly, no clinically significant changes were observed in vital signs, ECGs or urinalysis evaluations.[1]
|
||
| References |
|
||
| Additional Infomation |
Rilzabrutinib is an oral, reversible covalent inhibitor of Bruton's tyrosine kinase being investigated for the treatment of immune disorders, such as immune thrombocytopenic purpura.
Rilzabrutinib is an orally bioavailable reversible covalent inhibitor of Bruton's tyrosine kinase (BTK), with potential immunomodulatory and anti-inflammatory activities. Upon oral administration, rilzabrutinib inhibits the activity of BTK. This prevents the activation of the B-cell antigen receptor (BCR) signaling pathway, and the resulting immune activation and inflammation. BTK, a cytoplasmic tyrosine kinase and member of the Tec family of kinases, plays an important role in B-lymphocyte development, activation, signaling, proliferation and survival. In addition to B-cells, BTK is also expressed in other cells of hematopoietic origin, including monocytes, macrophages, neutrophils, mast cells, eosinophils and platelets, and plays an important role in both adaptive and innate immune responses. Drug Indication Treatment of immune thrombocytopenia |
| Molecular Formula |
C36H40FN9O3
|
|
|---|---|---|
| Molecular Weight |
665.7597
|
|
| Exact Mass |
665.32
|
|
| Elemental Analysis |
C, 64.95; H, 6.06; F, 2.85; N, 18.93; O, 7.21
|
|
| CAS # |
1575596-29-0
|
|
| Related CAS # |
1575596-77-8;1575596-29-0;1575591-66-0
|
|
| PubChem CID |
73388818
|
|
| Appearance |
White to off-white solid powder
|
|
| LogP |
3.4
|
|
| Hydrogen Bond Donor Count |
1
|
|
| Hydrogen Bond Acceptor Count |
11
|
|
| Rotatable Bond Count |
8
|
|
| Heavy Atom Count |
49
|
|
| Complexity |
1230
|
|
| Defined Atom Stereocenter Count |
1
|
|
| SMILES |
CC(C)(/C=C(\C#N)/C(=O)N1CCC[C@H](C1)N2C3=NC=NC(=C3C(=N2)C4=C(C=C(C=C4)OC5=CC=CC=C5)F)N)N6CCN(CC6)C7COC7
|
|
| InChi Key |
LCFFREMLXLZNHE-GBOLQPHISA-N
|
|
| InChi Code |
InChI=1S/C36H40FN9O3/c1-36(2,45-15-13-43(14-16-45)26-21-48-22-26)18-24(19-38)35(47)44-12-6-7-25(20-44)46-34-31(33(39)40-23-41-34)32(42-46)29-11-10-28(17-30(29)37)49-27-8-4-3-5-9-27/h3-5,8-11,17-18,23,25-26H,6-7,12-16,20-22H2,1-2H3,(H2,39,40,41)/b24-18+/t25-/m1/s1
|
|
| Chemical Name |
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (3.12 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (3.12 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 2.08 mg/mL (3.12 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.5020 mL | 7.5102 mL | 15.0204 mL | |
| 5 mM | 0.3004 mL | 1.5020 mL | 3.0041 mL | |
| 10 mM | 0.1502 mL | 0.7510 mL | 1.5020 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
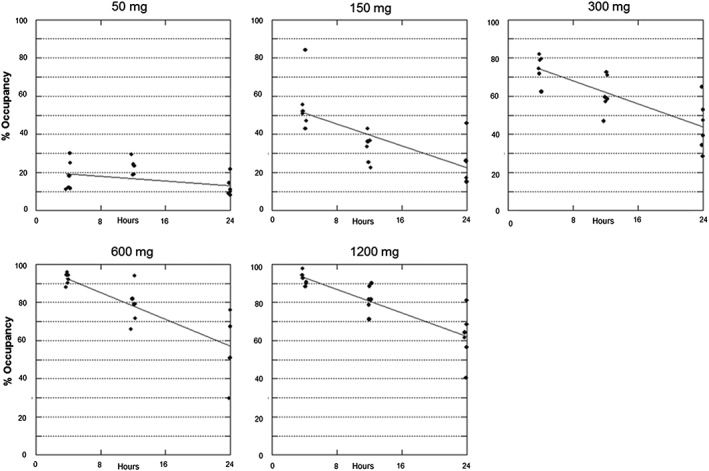 Individual BTK occupancy by PRN1008 dose level (Part A). Solid line represents fit of a linear regression model to estimate loss of occupancy over time.Br J Clin Pharmacol.2017 Nov;83(11):2367-2376. |
|---|
Duration of BTK occupancy (squares) in relation to the plasma concentration profile of PRN1008 (circles), following final dose on day 10 of a 600mg once daily dosing regimen in the multiple ascending dose study.Br J Clin Pharmacol.2017 Nov;83(11):2367-2376. |
Exposure–response relationship between 4‐hour BTK occupancy and PRN1008 maximum observed concentration (Part A).Br J Clin Pharmacol.2017 Nov;83(11):2367-2376. |