
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| Other Sizes |
Purity: ≥98%
PF-04691502 is a novel, potent, ATP-competitive and selective dual inhibitor of PI3K (phosphatidylinositol 3 kinase) and mTOR (mammalian target of rapamycin) with potential anticancer activity. With Ki of 1.8 nM, 2.1 nM, 1.6 nM, 1.9 nM, and 16 nM in cell-free assays, it inhibits PI3K(α/β/δ/γ)/mTOR. In terms of Vps34, AKT, PDK1, p70S6K, MEK, ERK, p38, or JNK, PF04691502 exhibits little activity. When cancer cells overexpress PI3K/mTOR, the potential anticancer drug PF-04691502 inhibits tumor growth.
| Targets |
PI3Kδ (Ki = 1.6 nM); PI3Kα (Ki = 1.8 nM); PI3Kγ (Ki = 1.9 nM); PI3Kβ (Ki = 2.1 nM); mTOR (Ki = 16 nM)
|
|---|---|
| ln Vitro |
PF-04691502 inhibits recombinant class I PI3K and mTOR in biochemical assays and prevents the transformation of avian fibroblasts caused by wild-type PI3K γ, δ, or mutant PI3Kα.
PF-04691502 inhibits cell proliferation (IC(50) of 179-313 nM) and lowers phosphorylation of AKT T308 and AKT S473 in PIK3CA-mutant and PTEN-deleted cancer cell lines, respectively. With an IC(50) of 32 nM, PF-04691502 inhibits the activity of mTORC1 in cells as determined by the PI3K-independent nutrient stimulated assay. It also prevents the activation of PI3K and mTOR downstream effectors such as AKT, FKHRL1, PRAS40, p70S6K, 4EBP1, and S6RP. While mTOR inhibition lasts for 24 to 48 hours after exposure to PF-04691502, short-term exposure primarily inhibits PI3K. In addition to upregulating p27 Kip1 and downregulating Rb, PF-04691502 causes cell cycle G(1) arrest. [1]
|
| ln Vivo |
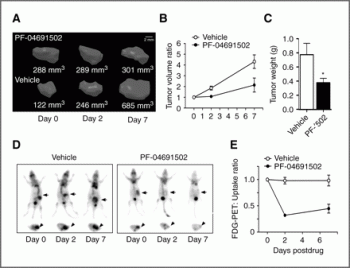
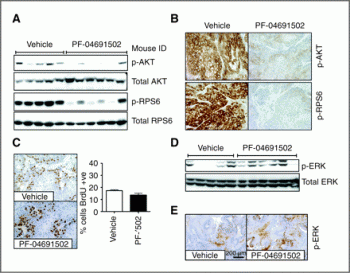
In SKOV3 (PIK3CA mutation), U87 (PTEN null), and gefitinib- and erlotinib-resistant non-small cell lung carcinoma xenografts, antitumor activity of PF-04691502 is seen. [1] At 7 days, PF-04691502 reduces tumor growth by 72%. FDG-PET imaging demonstrated that PF-04691502 significantly lowers glucose metabolism. Following PF-04691502 treatment, p-AKT (S473) and p-RPS6 (S240/244), two tissue biomarkers of PI3K/mTOR pathway activity, are also severely inhibited. [2]
|
| Enzyme Assay |
The following procedure is used for the ATP competitive inhibition fluorescence polarization assay: mPI3Kα dilution solution (90 nM) is prepared in fresh assay buffer (50 mM Hepes pH 7.4, 150 mM NaCl, 5 mM DTT, 0.05% CHAPS) and kept on ice. The enzyme reaction contains 0.5 nM mouse PI3Kα (p110α/p85α complex purified from insect cells), 30 μM PIP2, PF-04691502 (0, 1, 4, and 8 nM), 5 mM MgCl2, and 2-fold serial dilutions of ATP (0-800 μM). Dimethyl sulfoxide is 2.5% in the finished product. ATP is used to start the reaction, and 10 mM EDTA is used to stop it after 30 minutes. In a detection plate, 15 uL of the kinase reaction mixture is combined with 15 uL of the detector/probe mixture, which contains 480 nM GST-Grp1PH domain and 12 nM TAMRA-tagged fluorescent PIP3 in assay buffer. Before the plate is read on an LJL Analyst HT, it is shaken for 3 minutes and incubated for 35 to 40 minutes.
|
| Cell Assay |
In 96-well culture plates with growth medium containing 10% FBS, BT20, U87MG, and SKOV3 cells are seeded at a density of 3,000 cells per well. DMSO (0.1% final) or a compound that has been serially diluted is applied to cells after an overnight incubation period. 0.1 mg/mL receives resazurin addition. For three hours, plates are incubated in 5% CO2 at 37 °C. Following excitation at 530 nm, fluorescence signals are read as emission at 590 nm. Fluorescence intensity and drug concentration are plotted on a nonlinear curve to determine the IC50 values.
|
| Animal Protocol |
Mice: Female nu/nu mice (6-8 weeks old) are used. To prepare for implantation, tumor cells are removed and then re-suspended in matrigel (1:1) and serum-free medium. The region of the back flank is subcutaneously implanted with SKOV3, U87MG, or NSCLC cells (2.5-4 106). When a tumor's size ranges from 100 to 200 mm3, treatment can begin. The daily oral administration of PF-04691502 contains 0.5% methylcellulose in water suspension. Every two to three days, tumor volumes and animal body weights are measured. Vernier calipers are used to measure and calculate tumor volume. Calculated tumor growth inhibition (TGI) percentage. Data are shown as mean±SE.
|
| References | |
| Additional Infomation |
PF-04691502 is pI3K/mTOR Kinase Inhibitor PF-04691502 is an agent targeting the phosphatidylinositol 3 kinase (PI3K) and mammalian target of rapamycin (mTOR) in the PI3K/mTOR signaling pathway, with potential antineoplastic activity.
PF-04691502 has been used in trials studying the treatment of Cancer, Breast Neoplasms, Early Breast Cancer (Phase 2), and Advanced Breast Cancer (Phase 1b). PI3K/mTOR Kinase Inhibitor PF-04691502 is an agent targeting the phosphatidylinositol 3 kinase (PI3K) and mammalian target of rapamycin (mTOR) in the PI3K/mTOR signaling pathway, with potential antineoplastic activity. PI3K/mTOR kinase inhibitor PF-04691502 inhibits both PI3K and mTOR kinases, which may result in apoptosis and growth inhibition of cancer cells overexpressing PI3K/mTOR. Activation of the PI3K/mTOR pathway promotes cell growth, survival, and resistance to chemotherapy and radiotherapy; mTOR, a serine/threonine kinase downstream of PI3K, may also be activated independent of PI3K. Deregulation of the phosphoinositide 3-kinase (PI3K) signaling pathway such as by PTEN loss or PIK3CA mutation occurs frequently in human cancer and contributes to resistance to antitumor therapies. Inhibition of key signaling proteins in the pathway therefore represents a valuable targeting strategy for diverse cancers. PF-04691502 is an ATP-competitive PI3K/mTOR dual inhibitor, which potently inhibited recombinant class I PI3K and mTOR in biochemical assays and suppressed transformation of avian fibroblasts mediated by wild-type PI3K γ, δ, or mutant PI3Kα. In PIK3CA-mutant and PTEN-deleted cancer cell lines, PF-04691502 reduced phosphorylation of AKT T308 and AKT S473 (IC(50) of 7.5-47 nmol/L and 3.8-20 nmol/L, respectively) and inhibited cell proliferation (IC(50) of 179-313 nmol/L). PF-04691502 inhibited mTORC1 activity in cells as measured by PI3K-independent nutrient stimulated assay, with an IC(50) of 32 nmol/L and inhibited the activation of PI3K and mTOR downstream effectors including AKT, FKHRL1, PRAS40, p70S6K, 4EBP1, and S6RP. Short-term exposure to PF-04691502 predominantly inhibited PI3K, whereas mTOR inhibition persisted for 24 to 48 hours. PF-04691502 induced cell cycle G(1) arrest, concomitant with upregulation of p27 Kip1 and reduction of Rb. Antitumor activity was observed in U87 (PTEN null), SKOV3 (PIK3CA mutation), and gefitinib- and erlotinib-resistant non-small cell lung carcinoma xenografts. In summary, PF-04691502 is a potent dual PI3K/mTOR inhibitor with broad antitumor activity. PF-04691502 has entered phase I clinical trials.[1] The phosphatidylinositol 3-kinase (PI3K)/Akt pathway is commonly dysregulated in human cancer, making it an attractive target for novel anticancer therapeutics. We have used a mouse model of ovarian cancer generated by Kras(G12D) activation and Pten deletion in the ovarian surface epithelium for the preclinical assessment of a novel PI3K/mTOR inhibitor PF-04691502. To enable higher throughput studies, we developed an orthotopic primary transplant model from these mice and evaluated therapeutic response to PF-04691502 using small-animal ultrasound and FDG-PET imaging. PF-04691502 inhibited tumor growth at 7 days by 72% ± 9. FDG-PET imaging revealed that PF-04691502 reduced glucose metabolism dramatically, suggesting FDG-PET may be exploited as an imaging biomarker of target inhibition by PF-04691502. Tissue biomarkers of PI3K/mTOR pathway activity, p-AKT (S473), and p-RPS6 (S240/244), were also dramatically inhibited following PF-04691502 treatment. However, as a single agent, PF-04691502 did not induce tumor regression and the long-term efficacy was limited, with tumor proliferation continuing in the presence of drug treatment. We hypothesized that tumor progression was because of concomitant activation of the mitogen-activated protein kinase pathway downstream of Kras(G12D) expression promoting cell survival and that the therapeutic effect of PF-04691502 would be enhanced by combinatory inhibition of MEK using PD-0325901. This combination induced striking tumor regression, apoptosis associated with upregulation of Bim and downregulation of Mcl-1, and greatly improved duration of survival. These data suggest that contemporaneous MEK inhibition enhances the cytotoxicity associated with abrogation of PI3K/mTOR signaling, converting tumor growth inhibition to tumor regression in a mouse model of ovarian cancer driven by PTEN loss and mutant K-Ras. [1] The role of PI3K and MAPK pathways in tumor initiation and progression is well established; hence, several inhibitors of these pathways are currently in different stages of clinical trials. Recent studies identified a PI3K/mTOR (PF-04691502) and a MEK inhibitor (PD-0325901) with strong potency and efficacy in different cell lines and tumor models. PD-0325901, however, showed adverse effects when administered at or above MTD (maximum tolerated dose) in the clinic. Here, we show in preclinical models that PD-0325901 at doses well below MTD (sub-MTD 1.5 mg/kg SID) is still a potent compound as single agent or in combination with PF-04691502. We first observed that PD-0325901 at 1.5 mg/kg SID and in combination with PF-04691502 (7.5 mg/kg; SID) significantly inhibited growth of H460 (carry Kras and PIK3CA mutations) orthotopic lung tumors. Additionally, we tested efficacy of PD-0325901 in Kras(G12D-LSL) conditional GEMMs (genetically engineered mouse models) which are a valuable tool in translational research to study tumor progression. Intranasal delivery of adenoviruses expressing Cre recombinase (Adeno-Cre) resulted in expression of mutant Kras leading to development of tumor lesions in lungs including adenomatous hyperplasia, large adenoma, and adenocarcinoma. Similar to H460 tumors, PD-0325901 as single agent or in combination with PF-04691502 significantly inhibited growth of tumor lesions in lungs in Kras(G12D-LSL) mice when treatment started at adenocarcinoma stage (at 14 weeks post-Adeno-Cre inhalation). In addition, immunohistochemistry showed inhibition of pS6 (phosphorylated ribosomal S6) in the treated animals particularly in the combination group providing a proof of mechanism for tumor growth inhibition. Finally, m-CT imaging in live Kras(G12D-LSL) mice showed reduction of tumor burdens in PD-0325901-treated animals at sub-MTD dose. In conclusion, our data suggest that PD-0325901 at doses below MTD is still a potent compound capable of tumor growth inhibition where Kras and/or PI3K are drivers of tumor growth and progression. [3] |
| Molecular Formula |
C22H27N5O4
|
|
|---|---|---|
| Molecular Weight |
425.48
|
|
| Exact Mass |
425.206
|
|
| Elemental Analysis |
C, 62.10; H, 6.40; N, 16.46; O, 15.04
|
|
| CAS # |
1013101-36-4
|
|
| Related CAS # |
|
|
| PubChem CID |
25033539
|
|
| Appearance |
Off-white to gray solid powder
|
|
| Density |
1.4±0.1 g/cm3
|
|
| Boiling Point |
682.5±65.0 °C at 760 mmHg
|
|
| Flash Point |
366.5±34.3 °C
|
|
| Vapour Pressure |
0.0±2.2 mmHg at 25°C
|
|
| Index of Refraction |
1.646
|
|
| LogP |
1.43
|
|
| Hydrogen Bond Donor Count |
2
|
|
| Hydrogen Bond Acceptor Count |
8
|
|
| Rotatable Bond Count |
6
|
|
| Heavy Atom Count |
31
|
|
| Complexity |
654
|
|
| Defined Atom Stereocenter Count |
0
|
|
| SMILES |
O(C([H])([H])C([H])([H])O[H])C1([H])C([H])([H])C([H])([H])C([H])(C([H])([H])C1([H])[H])N1C(C(C2=C([H])N=C(C([H])=C2[H])OC([H])([H])[H])=C([H])C2=C(C([H])([H])[H])N=C(N([H])[H])N=C12)=O
|
|
| InChi Key |
XDLYKKIQACFMJG-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C22H27N5O4/c1-13-17-11-18(14-3-8-19(30-2)24-12-14)21(29)27(20(17)26-22(23)25-13)15-4-6-16(7-5-15)31-10-9-28/h3,8,11-12,15-16,28H,4-7,9-10H2,1-2H3,(H2,23,25,26)
|
|
| Chemical Name |
2-amino-8-[4-(2-hydroxyethoxy)cyclohexyl]-6-(6-methoxypyridin-3-yl)-4-methylpyrido[2,3-d]pyrimidin-7-one
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (5.88 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (5.88 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 2.5 mg/mL (5.88 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3503 mL | 11.7514 mL | 23.5029 mL | |
| 5 mM | 0.4701 mL | 2.3503 mL | 4.7006 mL | |
| 10 mM | 0.2350 mL | 1.1751 mL | 2.3503 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
Kinross KM, Mol Cancer Ther, 2011, 10(8), 1440-1449 |
 |
 |