
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| 1g | |||
| Other Sizes |
Purity: ≥98%
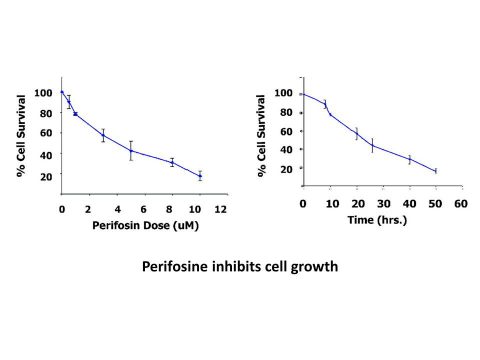
Perifosine (also known as KRX-0401) is a potent, orally bioavailable and synthetic antitumor alkylphospholipid (APL) Perifosine (also known as KRX-0401) is a potent, orally bioavailable and synthetic antitumor alkylphospholipid (APL) which acts as an Akt inhibitor and a PI3K inhibitor with potential anticancer activity. As a drug candidate, it was being developed for treating a variety of cancer such as multiple myeloma and neuroblastoma. In March 2013, Aeterna Zentaris announced the discontinuing of Phase 3 clinical trial of perifosine for the treatment of relapsed and refractory multiple myeloma. Perifosine induces cell apoptosis through inhibiting the activity of Akt. Perifosine shows antitumor activity in various cell lines including NSCLC, MM, epithelial carcinoma, prostate carcinoma and leukemia cells. In H460 cells, perifosine decreased cell survival and induced apoptosis with IC50 values of 1μM and 10 μM, respectively. The treatment of perifosine was also found to induce cleavage of caspase-8, caspase-9, caspase-3 and PARP in this cell line. In MM.1S cells, perifosine induced sub-G1 phase population increase from 15% to 57% at 10 μM and induced cleavage of caspase-8, caspase-9 and PARP in a dose-dependent manner. In MM.1S cells, perifosine induced sub-G1 phase population increase from 15% to 57% at 10 μM and induced cleavage of caspase-8, caspase-9 and PARP in a dose-dependent manner.
| Targets |
Akt (IC50 = 4.7 μM)
|
|---|---|
| ln Vitro |
Perifosine exhibits anti-proliferative properties in immortalized keratinocytes (HaCaT) and head and neck squamous carcinoma cells, with an IC50 range of 0.6 to 8.9 M. [1] Perifosine induces cell cycle arrest in G1 and G2, significantly lowers Akt and extracellular signal-regulated kinase (Erk) 1/2 phosphorylation levels, and inhibits the growth of mouse glial progenitors in a dose-dependent manner.[2] In MM.1S cells, periforosine (10 μM) completely prevents the phosphorylation of Akt.[3] A recent study shows Perifosine blocks Akt phosphorylation, causing cell cycle arrest and apoptosis in human hepatocellular carcinoma cell lines.[4]
|
| ln Vivo |
In vivo, the combination of perifosine and temozolomide inhibits the growth of tumors (a PDGF-driven gliomagenesis). According to the findings, perifosine is a useful medication for treating gliomas where the Akt and Ras-Erk 1/2 pathways are frequently activated. This suggests that perifosine may be a new candidate for treating gliomas in the clinic.[2] When compared to control animals given only PBS vehicle treatment, oral daily and weekly administration of Perifosine significantly reduces the growth of human MM tumors and increases survival.[3] Perifosine causes apoptosis in myeloma xenografts while inducing thrombocytosis, leukocytosis, and increasing myelopoiesis in murine marrow and spleen.[5]
|
| Enzyme Assay |
Perifosine (5 μM, 6 hours) is either present or absent during the culture of MM.1S cells.Following this, IL-6 (20 ng/mL, 10 minutes) is used to stimulate the cells. The Akt Kinase Assay Kit is then used to perform an in vitro akt kinase assay.
|
| Cell Assay |
The medium contains 10% FCS and the indicated concentration of Periosine, and the cells are incubated for 48 hours. The MTT assay uses 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide to measure the viability of cells. Roche's Cell Proliferation Kit I. With the help of a 96-well plate reader, the absorbance at 590 nm is measured.
|
| Animal Protocol |
Mice: Mice with tumors are given medication. Image-positive For 3 to 5 days, Ef-luc Ntv-a mice are given daily treatments that include intraperitoneal administration of buffer alone as a control, intraperitoneal administration of 100 mg/kg Temozolomide, oral administration of 30 mg/kg Perifosine, or a combination of Perifosine and Temozolomide. Treatments' average doses are as follows: Control, 5 (all five); Temozolomide, 3.75 (three to five); Perifosine, 3.75 (three to four); and Perifosine+Temozolomide, 3 (all three). In the control buffer solution, distilled water was combined with 5% DMSO and 1% Tween 80.
Rats: Rats are treated with Perifosine (20 mg/kg, ip, once), an Akt inhibitor, 30 min before rapamycin administration to further ascertain whether the paradoxical effect of rapamycin on S6 phosphorylation is connected to upstream signals of Akt-mTOR. Rats are euthanized 1 or 6 hours after receiving rapamycin injections. |
| References | |
| Additional Infomation |
Perifosine is a phospholipid consisting of 1,1-dimethylpiperidinium-4-yl hydrogen phosphate in which the hydrogen is replaced by a stearyl (octadecyl) group. It has a role as an EC 2.7.1.137 (phosphatidylinositol 3-kinase) inhibitor. It is a phospholipid and an ammonium betaine. It is functionally related to an octadecan-1-ol.
Perifosine is a novel alkylphospholipid with antiproliferative properties attributed to protein kinase B inhibition. Perifosine is an orally active alkyl-phosphocholine compound with potential antineoplastic activity. Targeting cellular membranes, perifosine modulates membrane permeability, membrane lipid composition, phospholipid metabolism, and mitogenic signal transduction, resulting in cell differentiation and inhibition of cell growth. This agent also inhibits the anti-apoptotic mitogen-activated protein kinase (MAPK) pathway and modulates the balance between the MAPK and pro-apoptotic stress-activated protein kinase (SAPK/JNK) pathways, thereby inducing apoptosis. Perifosine has a lower gastrointestinal toxicity profile than the related agent miltefosine. (NCI04) Drug Indication Investigated for use/treatment in solid tumors, multiple myeloma, leukemia (unspecified), lung cancer, and brain cancer. Mechanism of Action Targeting cellular membranes, perifosine modulates membrane permeability, membrane lipid composition, phospholipid metabolism, and mitogenic signal transduction, resulting in cell differentiation and inhibition of cell growth. This agent also inhibits the anti-apoptotic mitogen-activated protein kinase (MAPK) pathway and modulates the balance between the MAPK and pro-apoptotic stress-activated protein kinase (SAPK/JNK) pathways, thereby inducing apoptosis. |
| Molecular Formula |
C25H52NO4P
|
|---|---|
| Molecular Weight |
461.6584
|
| Exact Mass |
461.363
|
| Elemental Analysis |
C, 65.04; H, 11.35; N, 3.03; O, 13.86; P, 6.71
|
| CAS # |
157716-52-4
|
| Related CAS # |
157716-52-4
|
| PubChem CID |
148177
|
| Appearance |
White to off-white solid powder
|
| Melting Point |
271-272° (dec)
|
| LogP |
5.6
|
| Hydrogen Bond Donor Count |
0
|
| Hydrogen Bond Acceptor Count |
4
|
| Rotatable Bond Count |
20
|
| Heavy Atom Count |
31
|
| Complexity |
454
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
P(=O)([O-])(OC([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H])OC1([H])C([H])([H])C([H])([H])[N+](C([H])([H])[H])(C([H])([H])[H])C([H])([H])C1([H])[H]
|
| InChi Key |
SZFPYBIJACMNJV-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C25H52NO4P/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-24-29-31(27,28)30-25-20-22-26(2,3)23-21-25/h25H,4-24H2,1-3H3
|
| Chemical Name |
1,1-dimethylpiperidin-1-ium-4-yl octadecyl phosphate.
|
| Synonyms |
Perifosine; KRX-0401; KRX 0401; KRX0401; NKA17; NSC639966; NSC 639966; NSC-639966; D 21266; D-21266
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
DMSO: ~15 mg/mL (~32.5 mM)
Water: ~8 mg/mL (~17.3 mM) Ethanol: <1 mg/mL |
|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: 50 mg/mL (108.30 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with sonication.
Solubility in Formulation 2: 30%Propylene glycol, 5%Tween 80, 65% D5W: 30mg/mL (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1661 mL | 10.8305 mL | 21.6610 mL | |
| 5 mM | 0.4332 mL | 2.1661 mL | 4.3322 mL | |
| 10 mM | 0.2166 mL | 1.0830 mL | 2.1661 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Status | Interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01224730 | Completed | Drug: perifosine | Cancer | AEterna Zentaris | January 24, 2012 | Phase 1 |
| NCT01048580 | Completed | Drug: Perifosine Drug: Capecitabine |
Colon Cancer | AEterna Zentaris | October 2009 | Phase 1 |
| NCT00590954 | Completed | Drug: Perifosine | Malignant Gliomas CNS |
Memorial Sloan Kettering Cancer Center |
May 2006 | Phase 2 |
| NCT00498966 | Completed | Drug: Perifosine | Kidney Cancer | AEterna Zentaris | July 2007 | Phase 2 |
| NCT00375791 | Completed | Drug: perifosine Drug: dexamethasone |
Multiple Myeloma | AEterna Zentaris | December 2005 | Phase 2 |
|
 |