
| Size | Price | Stock | Qty |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g | |||
| Other Sizes |
Purity: =99.01%
Nintedanib (formerly BIBF-1120; trade name: Vargatef) is an orally bioavailable multi-kinase inhibitor with potential antineoplastic and anti-fibrotic activity. In cell-free experiments, it suppresses VEGFR1/2/3, FGFR1/2/3, and PDGFRα/β with IC50s of 34 nM/13 nM/13 nM, 69 nM/37 nM/108 nM, and 59 nM/65 nM. The FDA approved nitedanib in November 2014 to treat idiopathic pulmonary fibrosis (IPF).
| Targets |
VEGFR1 (IC50 = 34 nM); VEGFR2 (IC50 = 13 nM); VEGFR3 (IC50 = 13 nM); FGFR1 (IC50 = 69 nM); FGFR2 (IC50 = 37 nM); FGFR3 (IC50 = 108 nM); PDGFRα (IC50 = 59 nM); PDGFRβ (IC50 = 65 nM)
|
|---|---|
| ln Vitro |
Nintedanib (BIBF 1120) attaches itself to the ATP-binding site of the kinase domain, which is located in the cleft between the amino and carboxy terminal lobes. With an EC50 of 79 nM in cell assays, neintedanib (BIBF 1120) inhibits the proliferation of PDGF-BB stimulated BRPs. After stimulation with 5% serum plus PDGF-BB, neintedanib (BIBF 1120) (100 nM) inhibits MAPK activation. In cultures of human vascular smooth muscle cells (HUASMC), neintedanib (BIBF 1120) inhibits PDGF-BB stimulated proliferation with an EC50 of 69 nM[1].
Kinase selectivity profile. [1] Extensive biochemical testing revealed a distinctive, narrow range of kinases that are inhibited by Nintedanib/BIBF 1120 at pharmacologically relevant concentrations. The targeted kinases include all three VEGFR subtypes (IC50, 13–34 nmol/L), PDGFRα and PDGFRβ (IC50, 59 and 65 nmol/L), and FGFR types 1, 2, and 3 (IC50, 69, 37, and 108 nmol/L, respectively; Table 1). Comparable inhibition was seen for the corresponding human and rodent kinases. In addition, BIBF 1120 inhibits FLT3 (inhibition of acute myelogenous leukemia cell proliferation has been shown previously; ref. 29), as well as members of the Src-family (Src, Lyn, and Lck). By contrast, receptor tyrosine kinases, such as EGFR and HER2, InsR, IGF-IR, or the cell cycle kinases CDK1, CDK2, and CDK4 (Table 1) were not inhibited at concentrations below 1,000 nmol/L. Signaling pathways, proliferation, and survival of endothelial cells. Treatment of VEGF-stimulated endothelial cells derived from umbilical veins (HUVEC) and skin microvessels (HSMEC) with NintedanibBIBF 1120 resulted in inhibition of cell proliferation and apoptosis (EC50, <10 nmol/L; Table 2) and was preceded by inhibition of MAPK and Akt phosphorylation (Fig. 2A). Inhibition of bFGF-stimulated HUVEC proliferation required higher drug concentrations (EC50, 290 nmol/L), although activation of both MAPK and Akt was at least partially suppressed at concentrations down to 100 nmol/L. The apoptosis marker cleaved caspase-3 was up-regulated in a concentration-dependent manner in both VEGF-stimulated and bFGF-stimulated HUVEC, and the proportion of apoptotic HUVEC cells as measured by TUNEL stain increased from 2% in control cells to 28% in the presence of 50 nmol/L BIBF 1120 (Supplementary Fig. S1A). Effects on pericytes and smooth muscle cells. [1] Pericytes, important for vessel maturation and stabilization, are known to express PDGFRs (30). Nintedanib/BIBF 1120 inhibited proliferation of PDGF-BB–stimulated BRPs with an EC50 of 79 nmol/L (Table 2), which is in general agreement with the biochemical kinase inhibition data. Signaling pathway analysis showed that activation of MAPK after stimulation with 5% serum plus PDGF-BB could be blocked by BIBF 1120 at concentrations down to 100 nmol/L. Stimulation of BRP with 5% serum plus bFGF blocked MAPK phosphorylation, but not concentration-dependently (Fig. 2B). Activation of Akt was clearly suppressed by BIBF 1120 after stimulation with PDGF-BB or bFGF down to a concentration of 100 nmol/L; interestingly, no increase in cleaved caspase-3 resulted from this pathway inhibition. In cultures of human vascular smooth muscle cells (HUASMC), Nintedanib/BIBF 1120 inhibited PDGF-BB stimulated proliferation with an EC50 of 69 nmol/L (Table 2), and MAPK activation was inhibited at concentrations down to 100 nmol/L. Cell lysates of HUASMC stimulated with bFGF showed inhibition of MAPK activation above concentrations of 300 nmol/L. Phosphorylation of Akt was completely blocked in bFGF or PDGF-BB stimulated HUASMC at BIBF 1120 concentrations as low as 100 nmol/L. Furthermore, the apoptosis marker cleaved caspase-3 was up-regulated in bFGF-stimulated HUASMC treated with BIBF 1120 (Fig. 2C). Sustained VEGFR blockade. [1] To determine the duration of VEGFR-2 inhibition by Nintedanib/BIBF 1120, a pulse-chase experiment with VEGFR-2 transfected NIH3T3 cells (31) was performed. The cells were exposed for 1 hour to 50 nmol/L BIBF 1120, washed thoroughly with PBS, and incubated for 8, 24, or 32 hours in medium followed by stimulation with VEGF for 10 minutes. Western blot analysis of the cell lysates after immunoprecipitation revealed that inhibition of receptor phosphorylation was sustained for at least 32 hours after removal of BIBF 1120 (Supplementary Fig. S1B). Combination effect of trifluridine and Nintedanib on colorectal cancer cell lines in vitro [2] The isobologram plots were drawn using three isoeffect curves (mode I, mode IIa, and mode IIb) based on the 72-h growth inhibition curves for DLD-1, HT-29, and HCT116 cells (Fig. 1A-C) with trifluridine or nintedanib alone. Based on available dose-response curves, we analyzed the combined effect of the two drugs at the points of IC50. The IC50 values for trifluridine in DLD-1, HT-29, and HCT116 cells were 4.3×10−6, 3.8×10−6, and 1.8×10−6 M respectively, whereas the corresponding IC50 values for nintedanib were 3.4×10−6, 1.4×10−6 and 2.5×10−6 M, respectively. In the DLD-1 and HT-29 cells, a 72-h exposure to the combination treatment resulted in an additive effect (Fig. 1A and B). In the HCT116 cells the aforementioned combination treatment resulted in a sub-additive effect (Fig. 1C). |
| ln Vivo |
Nintedanib (BIBF 1120) 25–100 mg/kg daily p.o. is very active in all tumor models, including a syngeneic rat tumor model and human tumor xenografts growing in nude mice. This is demonstrated by the tumor's perfusion on magnetic resonance imaging after three days, its decreased vessel integrity and density after five days, and its significant growth inhibition[1]. Orally administered nitedanib (BIBF 1120) is well tolerated and shows encouraging efficacy in in vivo tumor models[2].
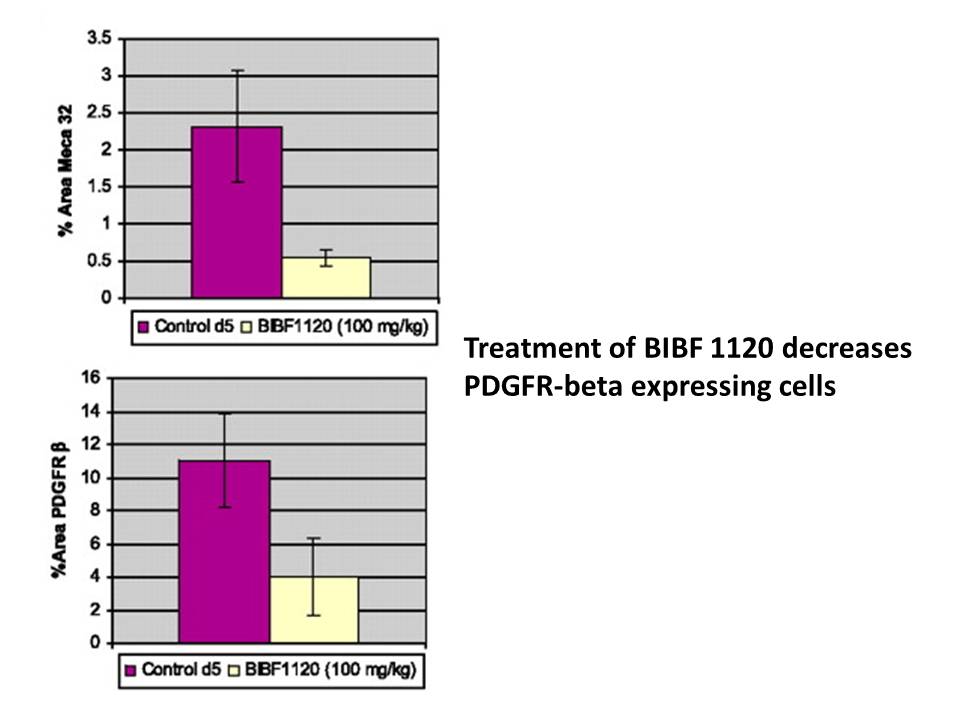
BIBF 1120/Nintedanib affects tumor vessel density and pericytes. [1] To confirm that BIBF 1120 affects the tumor vasculature, mice with established FaDu xenografts were treated for five consecutive days with either the vehicle control or BIBF 1120 at a dose of 100 mg/kg. After the last application, tumors were dissected and analyzed by immunohistochemistry using Meca 32 and PDGFRβ-specific antibodies to stain endothelial cells and pericytes (Fig. 3B). In comparison to control tumors, vessel density in xenografts from mice treated with BIBF 1120 was reduced by 76% (Fig. 3C; P < 0.001). Quantification of PDGFRβ-positive mural cells showed a reduction of 64% after 5 days of treatment with BIBF 1120 (Fig. 3C; P < 0.001). Double immunofluorescence staining with Meca 32 and PDGFRβ in tumor sections from control and BIBF 1120–treated mice show a clear association of Meca 32–positive endothelial cells and PDGFRβ-positive pericytes (Fig. 3D,, top) in the control mice, whereas in the BIBF 1120–treated mice, a marked reduction in both Meca 32–positive and PDGFRβ-positive cells was seen predominantly in the intratumoral compartment compared with the peritumoral tumor stroma separating the tumor nodules (Fig. 3D, area between the two dotted lines in the right bottom). At high magnification, a tight association between Meca 32–positive and PDGFRβ-positive cells can be seen in the tumor sample from a control mouse, but not in the BIBF 1120–treated tumor sample (Fig. 3D , arrow in left top and bottom). These data show not only the reduction of Meca 32–positive and PDGFRβ-positive cells upon BIBF 1120 treatment but also the loss of tight association between both cell types in the majority of the tumor vessels identified after 5 days of treatment. In vivo antitumor activity associated with distinctive pharmacokinetic profile and favorable tolerability in mice. [1] Continuous once daily p.o. treatment of mice with established FaDu tumor xenografts at 50 or 100 mg/kg resulted in a significant inhibition of tumor growth and treated versus control (T/C) values of 27% and 11%, respectively (Fig. 4A). BIBF 1120/Nintedanib was well tolerated even in the high-dose group, with no obvious weight loss over the treatment period. Marked inhibition of tumor growth was also observed in xenograft models of human renal cell carcinoma (Fig. 4B; Caki-1), colorectal (HT-29), ovarian (SKOV-3), non–small cell lung (Calu-6), and prostate carcinoma (PAC-120), as described in Supplementary Table S1. Moreover, in a syngeneic rat glioblastoma model (cell line GS-9L), efficacy was observed at 50, 25, and 10 mg/kg with T/C values of 30%, 45%, and 74%, respectively (Supplementary Table S1). Pharmacokinetic studies after p.o. application to mice (Fig. 4C) revealed a maximal plasma concentration of ∼1,000 nmol/L at 1 hour and trough plasma levels below 8 nmol/L at 24 hours postadministration. This distinctive pharmacokinetic profile can be explained by the rapid metabolization of BIBF 1120 by methyl ester cleavage, resulting in the generation of the main metabolite BIBF 1202 containing a free acid residue (data not shown). Antitumor efficacy of TFTD/Nintedanib combination therapy in vivo [2] The in vivo efficacy of TFTD monotherapy, Nintedanib monotherapy, and TFTD and nintedanib combination in human colorectal cancer xenograft models was evaluated. Nude mice bearing DLD-1 tumors were treated with 150 mg/kg TFTD, 40 mg/kg Nintedanib, or a combination of TFTD and nintedanib for 14 consecutive days. On day 15, TFTD monotherapy and nintedanib monotherapy resulted in a significant reduction in tumor growth in vivo (P<0.01) (Fig. 2A). In addition, the combination therapy exhibited greater antitumor activity than both monotherapies. The efficacy of the aforementioned treatments was evaluated in nude mice bearing tumors that were derived from 5-FU-resistant human colorectal cancer cells, DLD-1/5-FU (Fig. 2C). TFTD monotherapy and nintedanib monotherapy resulted in a significant reduction in tumor growth in vivo (P<0.01). The antitumor efficacy of both monotherapies was similar between the 5-FU-resistant DLD-1 cells and the parent DLD-1 cells. This indicated that no cross-resistance had occurred between DLD-1/5-FU and either of the monotherapies. The TFTD/nintedanib combination therapy exhibited greater antitumor activity against DLD-1/5-FU compared with the antitumor activity exhibited by both monotherapies. Thus, the combination therapy showed a similar antitumor effect against the DLD-1/5-FU (tumor growth inhibition rate 72.8%) and the DLD-1 (tumor growth inhibition rate 61.5%) tumors (data not shown). The efficacy of the above treatments was further evaluated in the HT-29 (Fig. 2E) and HCT116 (Fig. 2G) xenograft models. TFTD and nintedanib monotherapies both significantly suppressed tumor growth when compared with control (P<0.01). The combination therapy significantly suppressed tumor growth when compared to each monotherapy (P<0.01). Fig. 3 summarizes the antitumor effects of the administered therapies as evaluated by the mean RTV at day 15. The antitumor activity of the TFTD/nintedanib combination therapy, for all human colorectal cancer xenografts, was significantly greater than that of either monotherapy (P<0.01). |
| Enzyme Assay |
In vitro kinase activity assays. [1]
The cytoplasmic tyrosine kinase domain of VEGFR-2 (residues 797–1355 according to sequence deposited in databank SWISS-PROT P35968) was cloned into pFastBac fused to GST and extracted as described in supplementary methods. Enzyme activity was assayed using standard conditions using a random polymer (Glu/Tyr 4:1) and in the presence of 100 μmol/L ATP (for details, see supplementary methods). For all other kinase assays, the entire cytoplasmic domains of the receptors (from the end of the transmembrane to the COOH terminus) were cloned into pFastBac vector containing GST and assayed under standard conditions. In Vitro VEGFR-2 Kinase Assay [3] The cytoplasmic kinase domain of VEGFR-2 (residues 797 to 1335 according to sequence deposited in databank SWISS-PROT P35968) was cloned into pFastBac fused to Glutathion-S-transferase (GST). The GST-fusion protein was expressed in SF-9 insect cells and extracted with HEPEX (20 mM HEPES pH 7.4, 100 mM NaCl, 10 mM ss-glycerophosphate, 10 mM para-nitro-phenylphosphate, 30 mM NaF, 5 mM EDTA, 5% glycerol, 1% Triton X-100, 1 mM Na3VO4, 0.1% SDS, 0.5 μg/mL pepstatin A, 2.5 μg/mL 3,4-dichloroisocoumarin, 2.5 μg/mL trans-epoxysuccinyl-l-leucyl-l-amido butane, aprotinin 20 KIU/mL, leupeptin 2 μg/mL, benzamidine 1 mM and 0.002% PMSF). Enzyme activity was assayed in the presence or absence of serial dilutions of the inhibitor performed in 25% DMSO. Each microtiter plate contained internal controls such as blank, maximum reaction, and historical reference compound. All incubations were conducted at room temperature on a rotation shaker. Ten μL of each inhibitor dilution was added to 10 μL of diluted kinase (0.8 μg/mL VEGFR-2, 10 mM Tris pH 7.5, 2 mM EDTA, 2 mg/mL BSA) and preincubated for 1 h. The reaction was started by addition of 30 μL of substrate mix containing 62.4 mM Tris pH 7.5, 2.7 mM DTT, 5.3 mM MnCl2, 13.3 mM Mg-acetate, 0.42 mM ATP, 0.83 mg/mL Poly-Glu-Tyr(4:1), and 1.7 μg/mL Poly-Glu-Tyr(4:1)-biotin and incubated for 1 h. The reaction was stopped by addition of 50 μL of 250 mM EDTA, 20 mM HEPES, pH 7.4. Then 90 μL of stopped solution was transferred to a streptavidin plate and incubated for 1−2 h. After three washes with PBS the EU-labeled antibody, PY20 was added (recommended dilution 1:2000 of 0.5 mg/mL labeled antibody in DELFIA assay buffer). Excessive detection antibody was removed by three washes of DELFIA washing buffer. Then 10 minutes before measurement on the multilabel reader VICTOR, each well was incubated with 100 μL of DELFIA enhancement solution. IC50 values were calculated by using a sigmoidal curve analysis program using the nonlinear regression analysis with variable slope. The pFastBac clone containing the cytoplasmic tyrosine kinase domain of VEGFR2 (residues 797–1355 based on the sequence deposited in databank SWISS-PROT P35968) is fused to GST and extracted. The assay of enzyme activity is conducted in 25% DMSO with or without serial dilutions of Nintedanib/BIBF1120. There are internal controls on every microtiter plate, including blank, maximum reaction, and historical reference compound. On a rotating shaker, all incubations are carried out at room temperature. One hour is spent preincubating 10 μL of diluted kinase (0.8 μg/mL VEGFR2, 10 mM Tris pH 7.5, 2 mM EDTA, and 2 mg/mL BSA) with 10 μL of each BIBF1120 dilution. Addition of 30 μL of substrate mix containing 13.3 mM Mg-acetate, 6.2.4 mM Tris pH 7.5, 2.7 mM DTT, 5.3 mM MnCl2, 0.42 mM ATP, 0.83 mg/mL Poly-Glu-Tyr(4:1), and 1.7 μg/mL Poly-Glu-Tyr(4:1)-biotin initiates the reaction, which is then incubated for one hour. 90 μL of the reaction mix is placed on a streptavidin plate and incubated for one to two hours. The reaction is stopped by adding 50 μL of 250 mM EDTA, 20 mM HEPES, and pH 7.4. PY20 is added (recommended dilution 1:2000 of 0.5 mg/mL labeled antibody in DELFIA assay buffer) following three PBS washes with the EU-labeled antibody. Three DELFIA washing buffer washes are used to get rid of extra detection antibody. The DELFIA enhancement solution (100 μL) is then incubated in each well 10 minutes prior to measurement on the multilabel reader. |
| Cell Assay |
For the assay, the cell lines BRP, HUASMC, and HUVEC are employed. The cultures are supplemented with BIBF1120 two hours prior to the addition of ligands. There are cell lysates produced. Standard SDS-PAGE techniques are used for western blotting, with 50–75 μg of protein loaded per lane. Improved chemiluminescence aids in detection. Monoclonal antibodies M3807 and M8159 are used to analyze total and phosphorylated mitogen-activated protein kinase (MAPK). The monoclonal antibody for phosphorylated Akt (Ser473) is used to analyze it, while the corresponding polyclonal antibody is used to detect total Akt. While a corresponding antibody is used to detect KDR (VEGFR2) protein, monoclonal antibodies are also utilized to detect cleaved caspase-3.
Inhibition of cell signaling cascades in drug-treated cells. [1] HUVEC, HUASMC, and BRP were cultured as described above. Two hours before the addition of ligands, Nintedanib/BIBF 1120 was added to the cultures. Cell lysates were generated according to standard protocols. Western blotting was done using standard SDS-PAGE methods, loading 50 to 75 μg of protein per lane, with detection by enhanced chemiluminescence. Total and phosphorylated mitogen-activated protein kinase (MAPK) was analyzed using monoclonal antibodies. Cytotoxicity assay and evaluation of the combination effect in vitro [2] The drug cytotoxicity was measured with the crystal violet assay. The cells (2,000–4,000) were cultured in a 96-well microplate with 100 µl medium per well for 24 h. Trifluridine and Nintedanib were dissolved at the concentrations of 10 mM in dimethyl sulfoxide and the corresponding solutions were prepared using the culture medium under aseptic conditions. A total of 100 µl of the drug solution (trifluridine: 0.18–10 µM; nintedanib: 0.18–10 µM) were added into the culture medium. Following incubation of the plates for 72 h, the culture medium was removed and the cells were fixed with 4% glutaraldehyde for 30 min. The fixed cells were stained with 0.1% crystal violet for 2 min and washed and dissolved in 0.05 M NaH2PO4/50% ethanol. The absorbance was measured at a wavelength of 540 nm using a microplate reader. The cytotoxic effects of the trifluridine and Nintedanib combination were analyzed using the isobologram method. A total of 3 isoeffect curves (modes I, IIa, and IIb), based on the growth inhibition curves of trifluridine alone and nintedanib alone, were drawn. The total area enclosed by the three curves represented an ‘envelope of additivity’. The combination of drug treatment was considered to show a supra-additive (synergistic) interaction, when the experimentally observed IC50 values were included in the left side of the envelope, whereas when the IC50 values were included in the envelope, the combination was considered as additive. The combination was considered to be sub-additive, when the IC50 values were included on the right side of the envelope and were within the dotted line square. Finally, when the IC50 values fell outside the square, the combination was considered to be protective. |
| Animal Protocol |
For the assay, athymic NMRI-nu/nu female mice weighing between 21 and 33 grams are five to six weeks old. Following their acclimation, mice are injected with 1 to 5×106 (in 100 μL) of SKOV-3, FaDu, Caki-1, H460, HT-29, or PAC-120 cells subcutaneously into their right flank. Following their acclimation, 5×106 (in 100 μL) GS-9L cells are subcutaneously injected into the right flank of F344 Fischer rats. Blood is extracted from the retroorbital plexus of mice at predetermined intervals for pharmacokinetic analysis, and plasma is examined using high performance liquid chromatography-mass spectrometry methodology[1].
In vivo tumor models.[1] Five-week-old to 6-wk-old athymic NMRI-nu/nu female mice (21–31 g) were used. After acclimatization, mice were inoculated with 1 to 5 × 106 (in 100 μL) FaDu, Caki-1, SKOV-3, H460, HT-29, or PAC-120 cells s.c. into the right flank of the animal. F344 Fischer rat were injected with 5 × 106 (in 100 μL) GS-9L cells s.c. into the right flank of the animal. For pharmacokinetic analysis, blood was isolated at indicated time points from the retroorbital plexus of mice and plasma was analyzed using high performance liquid chromatography–mass spectrometry methodology. [1] TFTD was prepared by mixing trifluridine and TPI at a molar ratio of 1:0.5 in 0.5% HPMC solution. The dose of TFTD was expressed on the basis of the trifluridine content. TFTD was administered orally from day 1 to 14, twice a day at 6-h intervals at the reported effective dose (150 mg/kg/day). Nintedanib was administered orally from day 1 to 14, twice a day at 6-h intervals at the reported effective dose (40 mg/kg/day) (14,24). The vehicle solution that consisted of 0.5% HPMC solution was administered at 10 ml/kg to the control mouse group, following the same administration schedules as for the test drugs [2]. |
| ADME/Pharmacokinetics |
Pharmacokinetic studies after p.o. application to mice (Fig. 4C) revealed a maximal plasma concentration of ∼1,000 nmol/L at 1 hour and trough plasma levels below 8 nmol/L at 24 hours postadministration. This distinctive pharmacokinetic profile can be explained by the rapid metabolization of Nintedanib/BIBF 1120 by methyl ester cleavage, resulting in the generation of the main metabolite BIBF 1202 containing a free acid residue (data not shown). [1]
Rapid in vivo effects on tumor perfusion and permeability detected by DCE-MRI. [1] Human FaDu (squamous cell carcinoma of the head and neck) xenografts growing in nude mice were analyzed by DCE-MRI using gadolinium contrast agent before and 72 hours after initiation of daily p.o. treatment with Nintedanib/BIBF 1120 at 100 mg/kg. Tumor perfusion and vascular permeability was readily visible in the initial MRI scan and clearly reduced after 3 days of treatment (Fig. 3A); quantitation of the KTRANS value showed a significant decrease in Nintedanib/BIBF 1120–treated tumors compared with both baseline values and untreated controls (Fig. 3A). Because of their cellular potency and attractive selectivity profiles, compounds 2 and 3 were selected for in vivo testing. Both compounds yielded good plasma levels 2 h after oral administration to mice and were almost completely cleared from plasma 24 h after administration (Table 3). As shown for the lead structures, none of the compounds inhibited the proliferation of VEGF-independent cell lines at similar concentrations to those tested in HUVEC cells (EC50 > 1 μM), particularly HeLa, Calu-6, and FaDu tumor cell lines. Preclinical Pharmacokinetics Relevant to Human Pharmacokinetics [4] The pharmacokinetics and drug metabolism of nintedanib (dosed via intravenous [IV] infusion or oral gavage) were studied in several animal species. Mean plasma protein binding of nintedanib was > 97% in mice and rats, 91–93% in monkeys, and 98% in humans, over a concentration range of 50–2000 ng/mL [13, 17, 31]. Albumin was the major binding protein. Following administration of [14C]-radiolabelled nintedanib to rats, radioactivity was widely distributed into most of the tissues (except the central nervous system [CNS]). Repeated oral dosing ([14C]-radiolabelled nintedanib 30 mg/kg) for 13 days showed a slight accumulation in some tissues, although a similar accumulation in plasma concentrations was not apparent. Clinical Pharmacokinetics [4] The clinical pharmacokinetics of nintedanib monotherapy were investigated in healthy subjects, volunteers with hepatic impairment, and in patients with IPF or various advanced types of cancer. In healthy volunteers, only single-dose administration was performed. Key pharmacokinetic parameters following single and steady-state twice-daily dosing of nintedanib in patients with advanced cancer are presented in Table 2. The pharmacokinetics of nintedanib were further characterised by two successive population pharmacokinetic (PopPK) analyses, the first based on combined pharmacokinetic data from NSCLC (n = 849) and IPF (n = 342) patients and the second in IPF patients only (n = 933) enrolled in the phase II and III trials. For comparison, Table 3 gives key pharmacokinetic parameters after multiple dosing of nintedanib to typical patients with IPF or NSCLC based on the PopPK analyses. These results show that the key pharmacokinetic parameters are consistent across the two patient populations. |
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No information is available on the clinical use of nintedanib during breastfeeding. Because nintedanib is more than 97% bound to plasma proteins, the amount in milk is likely to be low. However, its half-life is about 9.7 hours and it might accumulate in the infant. The manufacturer recommends that breastfeeding be discontinued during nintedanib therapy. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. In Vitro Drug–Drug Interaction Victim and Perpetrator Properties [4] Several in vitro metabolism, transport and drug interaction studies were performed to quantitatively assess the drug–drug interaction potential of nintedanib. In vitro studies with human hepatocytes and/or human liver microsomes showed that nintedanib is a minor substrate for cytochrome P450 (CYP) 3A4 isoenzyme and has a very low potential (along with its two major metabolites [BIBF 1202 and BIBF 1202 glucuronide]) to inhibit or induce CYP isoenzymes, including those that are most relevant or genetically polymorphic for drug metabolism in humans (CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19 and CYP3A4). In human liver microsomes, nintedanib was rapidly hydrolysed by esterases (major mechanism) and demethylated by CYP3A4 (minor mechanism) to form the metabolites BIBF 1202 and BIBF 1053, respectively. CYP-dependent metabolism accounted for about 5% compared to about 25% ester cleavage. Thus, drug–drug interactions with nintedanib as a victim of CYP enzyme-modulating agents (e.g. with co-medication with CYP inhibitors or inducers) are considered very unlikely. Furthermore, drug–drug interactions with nintedanib as a perpetrator of CYP enzymes (e.g. nintedanib acting as a CYP enzyme inhibitor or inducer) are also considered very unlikely. Further in vitro data indicated that nintedanib, at clinically relevant concentrations, did not inhibit glucuronidation by uridine 5′-diphospho-glucuronosyltransferase (UDP-glucuronosyltransferase, UGT) 1A1 (UGT1A1) in human liver microsomes. UGT1A1 is responsible for the glucuronidation of the metabolite BIBF 1202 to BIBF 1202 glucuronide in human liver microsomes. In addition, BIBF 1202 was glucuronidated by several intestinal UGTs (UGT1A7, UGT1A8, UGT1A10). A clinically relevant drug–drug interaction based on inhibition of UGT after oral administration of nintedanib is considered less likely as all half-maximal inhibitory concentration (IC50) values are substantially higher than the therapeutic plasma concentrations. In vitro assays using transfected MDCK cells demonstrated that nintedanib is a substrate of the efflux transporter P-gp and weakly inhibits P-gp (Table 1). Studies using cell lines that express different drug transporters showed that nintedanib was not a substrate of organic anion-transporting polypeptide (OATP) 1B1, OATP1B3, OATP2B1, organic cation transporter (OCT) 2, multidrug resistance-associated protein 2 (MRP-2) or the efflux breast cancer resistance protein (BCRP), but was a weak substrate for OCT1. Nintedanib did not inhibit OATP1B1-, OATP1B3-, OATP2B1-, OCT1-, OCT2-, P-gp- or BRCP-mediated transport at clinically relevant concentrations. |
| References |
|
| Additional Infomation |
Nintedanib is a member of the class of oxindoles that is a kinase inhibitor used (in the form of its ethylsulfonate salt) for the treatment of idiopathic pulmonary fibrosis and cancer. It has a role as an antineoplastic agent, a tyrosine kinase inhibitor, a vascular endothelial growth factor receptor antagonist, a fibroblast growth factor receptor antagonist and an angiogenesis inhibitor. It is an aromatic ester, a methyl ester, a member of oxindoles, an enamine, an aromatic amine, an aromatic amide and a N-alkylpiperazine. It is a conjugate base of a nintedanib(1+).
Nintedanib is a Kinase Inhibitor. The mechanism of action of nintedanib is as a Protein Kinase Inhibitor. See also: Nintedanib (annotation moved to). Drug Indication Ofev is indicated in adults for the treatment of Idiopathic Pulmonary Fibrosis (IPF). Inhibition of tumor angiogenesis through blockade of the vascular endothelial growth factor (VEGF) signaling pathway is a novel treatment modality in oncology. Preclinical findings suggest that long-term clinical outcomes may improve with blockade of additional proangiogenic receptor tyrosine kinases: platelet-derived growth factor receptors (PDGFR) and fibroblast growth factor receptors (FGFR). BIBF 1120 is an indolinone derivative potently blocking VEGF receptor (VEGFR), PDGFR and FGFR kinase activity in enzymatic assays (IC(50), 20-100 nmol/L). BIBF 1120 inhibits mitogen-activated protein kinase and Akt signaling pathways in three cell types contributing to angiogenesis, endothelial cells, pericytes, and smooth muscle cells, resulting in inhibition of cell proliferation (EC(50), 10-80 nmol/L) and apoptosis. In all tumor models tested thus far, including human tumor xenografts growing in nude mice and a syngeneic rat tumor model, BIBF 1120 is highly active at well-tolerated doses (25-100 mg/kg daily p.o.), as measured by magnetic resonance imaging of tumor perfusion after 3 days, reducing vessel density and vessel integrity after 5 days, and inducing profound growth inhibition. A distinct pharmacodynamic feature of BIBF 1120 in cell culture is sustained pathway inhibition (up to 32 hours after 1-hour treatment), suggesting slow receptor off-kinetics. Although BIBF 1120 is rapidly metabolized in vivo by methylester cleavage, resulting in a short mean residence time, once daily oral dosing is fully efficacious in xenograft models. These distinctive pharmacokinetic and pharmacodynamic properties may help explain clinical observations with BIBF 1120, currently entering phase III clinical development. [1] Trifluridine/tipiracil (TFTD) is a combination drug that is used for the treatment of metastatic colorectal cancer and was formerly known as TAS-102. It is a combination of two active pharmaceutical compounds, trifluridine, an antineoplastic thymidine-based nucleoside analog, and tipiracil, which enhances the bioavailability of trifluridine in vivo. TFTD is used for the treatment of patients with unresectable advanced or recurrent colorectal cancer that is resistant to standard therapies. In the present study, the anticancer effects of trifluridine in combination with nintedanib, an oral triple angiokinase inhibitor, on human colorectal cancer cell lines were investigated. The cytotoxicity against DLD-1, HT-29, and HCT116 cell lines was determined by the crystal violet staining method. The combination of trifluridine and nintedanib exerted an additive effect on the growth inhibition of DLD-1 and HT-29 cells and a sub-additive effect on HCT116 cells, as determined by isobologram analyses. Subsequently, the human colorectal cancer cell lines were implanted subcutaneously into nude mice to allow the evaluation of the in vivo tumor growth inhibitory effects of TFTD and nintedanib combination therapy. TFTD (150 mg/kg/day) and/or nintedanib (40 mg/kg/day) were orally administered to the mice twice daily from day 1 to day 14. The tumor growth inhibition with combination therapy was 61.5, 72.8, 67.6 and 67.5% for the DLD-1, DLD-1/5-FU, HT-29, and HCT116 xenografts, respectively. This was significantly (P<0.05) higher than the effects of monotherapy with either TFTD or nintedanib. These results demonstrated the effectiveness of the combination of TFTD and nintedanib in the treatment of colorectal cancer xenografts. The concentration of trifluridine incorporated into DNA in the HT-29 and HCT116 tumors was determined by liquid chromatography-tandem mass spectrometry. The incorporation levels following treatment with TFTD and nintedanib for 14 consecutive days were higher than those associated with TFTD treatment alone. The preclinical findings indicate that the combination therapy with TFTD and nintedanib is a promising treatment option for colorectal cancer. [2] Inhibition of tumor angiogenesis through blockade of the vascular endothelial growth factor (VEGF) signaling pathway is a new treatment modality in oncology. Preclinical findings suggest that blockade of additional pro-angiogenic kinases, such as fibroblast and platelet-derived growth factor receptors (FGFR and PDGFR), may improve the efficacy of pharmacological cancer treatment. Indolinones substituted in position 6 were identified as selective inhibitors of VEGF-, PDGF-, and FGF-receptor kinases. In particular, 6-methoxycarbonyl-substituted indolinones showed a highly favorable selectivity profile. Optimization identified potent inhibitors of VEGF-related endothelial cell proliferation with additional efficacy on pericyctes and smooth muscle cells. In contrast, no direct inhibition of tumor cell proliferation was observed. Compounds 2 (BIBF 1000) and 3 (BIBF 1120) are orally available and display encouraging efficacy in in vivo tumor models while being well tolerated. The triple angiokinase inhibitor 3 is currently in phase III clinical trials for the treatment of nonsmall cell lung cancer. [3] Nintedanib is an oral, small-molecule tyrosine kinase inhibitor approved for the treatment of idiopathic pulmonary fibrosis and patients with advanced non-small cell cancer of adenocarcinoma tumour histology. Nintedanib competitively binds to the kinase domains of vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF). Studies in healthy volunteers and in patients with advanced cancer have shown that nintedanib has time-independent pharmacokinetic characteristics. Maximum plasma concentrations of nintedanib are reached approximately 2-4 h after oral administration and thereafter decline at least bi-exponentially. Over the investigated dose range of 50-450 mg once daily and 150-300 mg twice daily, nintedanib exposure increases are dose proportional. Nintedanib is metabolised via hydrolytic ester cleavage, resulting in the formation of the free acid moiety that is subsequently glucuronidated and excreted in the faeces. Less than 1% of drug-related radioactivity is eliminated in urine. The terminal elimination half-life of nintedanib is about 10-15 h. Accumulation after repeated twice-daily dosing is negligible. Sex and renal function have no influence on nintedanib pharmacokinetics, while effects of ethnicity, low body weight, older age and smoking are within the inter-patient variability range of nintedanib exposure and no dose adjustments are required. Administration of nintedanib in patients with moderate or severe hepatic impairment is not recommended, and patients with mild hepatic impairment should be monitored closely and the dose adjusted accordingly. Nintedanib has a low potential for drug-drug interactions, especially with drugs metabolised by cytochrome P450 enzymes. Concomitant treatment with potent inhibitors or inducers of the P-glycoprotein transporter can affect the pharmacokinetics of nintedanib. At an investigated dose of 200 mg twice daily, nintedanib does not have proarrhythmic potential.[4] |
| Molecular Formula |
C31H33N5O4
|
|---|---|
| Molecular Weight |
539.62
|
| Exact Mass |
539.253
|
| Elemental Analysis |
C, 69.00; H, 6.16; N, 12.98; O, 11.86
|
| CAS # |
656247-17-5
|
| Related CAS # |
Nintedanib esylate;656247-18-6;Nintedanib-13C,d3;Nintedanib-d3;1624587-84-3;Nintedanib-d8;1624587-87-6
|
| PubChem CID |
135423438
|
| Appearance |
Yellow solid powder
|
| Density |
1.3±0.1 g/cm3
|
| Boiling Point |
742.2±60.0 °C at 760 mmHg
|
| Flash Point |
402.7±32.9 °C
|
| Vapour Pressure |
0.0±2.5 mmHg at 25°C
|
| Index of Refraction |
1.658
|
| LogP |
2.59
|
| Hydrogen Bond Donor Count |
2
|
| Hydrogen Bond Acceptor Count |
7
|
| Rotatable Bond Count |
8
|
| Heavy Atom Count |
40
|
| Complexity |
892
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
O=C(C([H])([H])N1C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])C1([H])[H])N(C([H])([H])[H])C1C([H])=C([H])C(=C([H])C=1[H])/N=C(\C1C([H])=C([H])C([H])=C([H])C=1[H])/C1=C(N([H])C2C([H])=C(C(=O)OC([H])([H])[H])C([H])=C([H])C1=2)O[H]
|
| InChi Key |
CPMDPSXJELVGJG-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C31H33N5O4/c1-34-15-17-36(18-16-34)20-27(37)35(2)24-12-10-23(11-13-24)32-29(21-7-5-4-6-8-21)28-25-14-9-22(31(39)40-3)19-26(25)33-30(28)38/h4-14,19,33,38H,15-18,20H2,1-3H3
|
| Chemical Name |
methyl 2-hydroxy-3-[N-[4-[methyl-[2-(4-methylpiperazin-1-yl)acetyl]amino]phenyl]-C-phenylcarbonimidoyl]-1H-indole-6-carboxylate
|
| Synonyms |
BIBF1120; Nintedanib; BIBF-1120; Intedanib; BIBF 1120; trade name: Vargatef
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: 10 mg/mL (18.53 mM) in 50% PEG300 50% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication.
Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 10 mg/mL (18.53 mM) in 1% CMC 0.5% Tween-80 (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. View More
Solubility in Formulation 3: 30% PEG400+0.5% Tween80+5% propylene glycol: 30mg/mL |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8532 mL | 9.2658 mL | 18.5316 mL | |
| 5 mM | 0.3706 mL | 1.8532 mL | 3.7063 mL | |
| 10 mM | 0.1853 mL | 0.9266 mL | 1.8532 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
Post-marketing Surveillance of Ofev Capsules in Chronic Fibrosing Interstitial Lung Diseases With a Progressive Phenotype in Japan
CTID: NCT04559581
Phase: Status: Active, not recruiting
Date: 2024-11-18
Cancer Res. 2008 Jun 15;68(12):4774-82. |
 |
Cancer Res. 2008 Jun 15;68(12):4774-82. |