
| Size | Price | Stock | Qty |
|---|---|---|---|
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
Purity: ≥98%
Mitoglitazone (MSDC-0160; CAY10415), a thiazolidinedione analog,is the prototypical mTOT(mitochondrial target of thiazolidinediones)-modulating insulin sensitizer being investigated to treat diabetes and Alzheimer's disease. It is an insulin sensitizer free of side effects that are dependent on the peroxisome proliferator-activated receptor-γ (PPAR-γ). Insulin content is preserved and the resistance of the insulin-signaling pathway is decreased when islets are co-incubated with MSDC-0160 and IGF-1. Significantly reducing the loss of insulin content is also prevented by MSDC-0160 at 50μM. In addition, MSDC-0160 treatment results in AMPK phosphorylation being increased while mTOR phosphorylation is decreased. Furthermore, it is discovered that MSDC-0160 exhibits pro-survival effects in human islets by raising the expression of apoptosis-related genes like bcl2 and decreasing the amount of cleaved caspase-3.
| Targets |
mTOT
|
|---|---|
| ln Vitro |
MSDC-0160 restores IGF-1-induced Akt and GSK-3 phosphorylation and lessens resistance in the insulin/IGF-1 signaling pathway. When combined with IGF-1 and 8 mM glucose, MSDC-0160 elevates the expression of insulin, pdx1, nkx6.1, and nkx2.2 genes specific to β-cells and preserves insulin levels without affecting glucose-stimulated insulin secretion. Additionally, MSDC-0160 decreases the expression of apoptosis markers and increases human β-cell differentiation.[1]
|
| ln Vivo |
MSDC-0160 (100 mg/kg p.o.) improves insulin sensitivity by causing a significant drop in the product of circulating insulin and glucose in diabetic KKAy mice.
Mitochondrial and autophagic dysfunction as well as neuroinflammation are involved in the pathophysiology of Parkinson's disease (PD). We hypothesized that targeting the mitochondrial pyruvate carrier (MPC), a key controller of cellular metabolism that influences mTOR (mammalian target of rapamycin) activation, might attenuate neurodegeneration of nigral dopaminergic neurons in animal models of PD. To test this, we used MSDC-0160, a compound that specifically targets MPC, to reduce its activity. MSDC-0160 protected against 1-methyl-4-phenylpyridinium (MPP+) insult in murine and cultured human midbrain dopamine neurons and in an α-synuclein-based Caenorhabditis elegans model. In 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice, MSDC-0160 improved locomotor behavior, increased survival of nigral dopaminergic neurons, boosted striatal dopamine levels, and reduced neuroinflammation. Long-term targeting of MPC preserved motor function, rescued the nigrostriatal pathway, and reduced neuroinflammation in the slowly progressive Engrailed1 (En1+/-) genetic mouse model of PD. Targeting MPC in multiple models resulted in modulation of mitochondrial function and mTOR signaling, with normalization of autophagy and a reduction in glial cell activation. Our work demonstrates that changes in metabolic signaling resulting from targeting MPC were neuroprotective and anti-inflammatory in several PD models, suggesting that MPC may be a useful therapeutic target in PD.[2] |
| Enzyme Assay |
Even at 50μM, MSDC-0160 considerably reduces the loss of insulin content. Furthermore, treatment of MSDC-0160 decreases mTOR phosphorylation while increasing AMPK phosphorylation. Additionally, MSDC-0160 is discovered to exhibit pro-survival effects in human islets by upregulating the expression of genes linked to apoptosis, such as bcl2, and lowering the quantity of cleaved caspase-3.
Binding of MSDC-0160 to its Mitochondrial Target Protein (mTOT)[1] Human islets (∼1000–1500/sample) were homogenized, and 10 µg of protein (mitochondrial P2 fraction) was incubated with a 125I-labeled probe (MSDC-1101) that binds to Mpc2 with or without the concomitant addition of 20 µM MSDC-0160 as previously described. |
| Cell Assay |
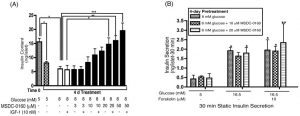
In human β-cells, MSDC-0160 inhibits nuclear translocation by localizing β-catenin on the cytoplasm or cell membrane. Islets were cultured in cCMRL with 8 mM glucose ± MSDC-0160 at 37°C. After 4 days, MSDC-0160 was removed and islets incubated for 90 min in cCMRL with 5 mM glucose. Insulin secretion was measured by static incubation for 30 min with cCMRL containing 5 or 16.5 mM glucose ± forskolin. Triplicate samples of 10 islets for each treatment were used. Media were collected and insulin determined by Human Insulin Specific RIA[1].
|
| Animal Protocol |
Ten- to 12-week-old male C57BL/6J mice weighing 24 to 28 g
30 mg/kg Oral gavage; daily; for 7 days C. elegans MPP+ neurodegeneration assay[2] Worms were synchronized, and L1 larvae (BY250 strain, GFP expression in dopaminergic neurons) were placed in 96-well plates containing OP50 bacteria in liquid culture (6 mg/ml) and MPP+ (0.75 mM) with MSDC-0160 (1, 10, or 100 μM). Wells containing no MPP+ [substituted with water or vehicle (methylcellulose)] and with MSDC-0160 alone were also included. Because in these experiments worms were soaked in solution containing MSDC-0160 of varying concentrations, the exact dose ingested by the worm cannot be accurately calculated. Therefore, the maximum concentration that had a neuroprotective effect was used in subsequent experiments to ensure efficacy. About 50 worms were added per well, and the plate was incubated at 20°C for 48 hours. After 48 hours, worms were moved and allowed to recover on unseeded nematode growth medium plates for several minutes before being mounted on an agarose pad with 3 mM levamisole and analyzed microscopically. Each worm was scored for the presence of GFP-fluorescent dopaminergic neurons in the anterior of the worm (CEP and ADE neurons). A minimum of 25 worms were analyzed for each condition in three independent trials. Measurement of brain and plasma drug concentrations[2] C57BL/6J mice were orally dosed with MSDC-0160 (30 mg/kg) suspended in 1% low-viscosity methylcellulose with 0.01% Tween 80 in distilled water (10 ml/kg) and then euthanized 2 and 4 hours later. Plasma and whole-brain homogenates were extracted and subjected to LC/mass spectrometry measurement of both active drug and active alcohol metabolite (MSDC-0037), as previously described. MPTP mouse model[2] Ten- to 12-week-old male C57BL/6J mice weighing 24 to 28 g were housed under standard conditions: constant temperature (22°C), humidity (relative, 30%), and a 12-hour light/dark cycle, with free access to food and water. Procedures were performed during daylight hours and were approved and supervised by the Institutional Animal Care and Use Committee at the Van Andel Research Institute. Mice were administered MSDC-0160 (30 mg/kg) by oral gavage beginning 24 hours before MPTP treatment. Next, mice received five consecutive doses of MPTP via intraperitoneal injection at 25 mg/kg per day (subacute regimen) along with coadministration of MSDC-0160, followed by 6 days of MSDC-0160 treatment. MSDC-0160 was dissolved in 1% methylcellulose with 0.01% Tween 80. Control mice received vehicle treatment (1% methylcellulose with 0.01% Tween 80). In the modest pathology stage, mice were administered MSDC-0160 (30 mg/kg per day) by oral gavage for 7 days starting 3 days after MPTP (25 mg/kg per day) treatment. Seven days after MPTP treatment, mice were euthanized, and tissues were processed for further evaluation. Mice were randomized to the experimental groups. En1+/− mouse model[2] The En1+/− heterozygous mice were maintained on an OF1 genetic background and under the same conditions as the C57BL/6J mice. In the mild pathology stage, En1+/− mice were fed a diet of chow formulated to deliver MSDC-0160 (30 mg/kg) starting at 3 weeks of age (at this point, no nigral cell death is detectable) and were euthanized at two different time points: 28 and 48 weeks. In the modest pathology stage, En1+/− mice were fed a diet of chow formulated to deliver v (30 mg/kg) starting at 8 weeks of age (at this point, 15 to 20% nigral cell death is detectable) and were euthanized at either 16 or 28 weeks. Mice were randomized to the groups, and control mice were fed regular chow. |
| References |
|
| Additional Infomation |
5-[[4-[2-(5-ethyl-2-pyridinyl)-2-oxoethoxy]phenyl]methyl]thiazolidine-2,4-dione is an aromatic ether.
Mitoglitazone has been used in trials studying the treatment of Type 2 Diabetes and Alzheimer's Disease. Major bottlenecks in the expansion of human β-cell mass are limited proliferation, loss of β-cell phenotype, and increased apoptosis. In our previous studies, activation of Wnt and mTOR signaling significantly enhanced human β-cell proliferation. However, isolated human islets displayed insulin signaling pathway resistance, due in part to chronic activation of mTOR/S6K1 signaling that results in negative feedback of the insulin signaling pathway and a loss of Akt phosphorylation and insulin content. We evaluated the effects of a new generation insulin sensitizer, MSDC-0160, on restoring insulin/IGF-1 sensitivity and insulin content in human β-cells. This novel TZD has low affinity for binding and activation of PPARγ and has insulin-sensitizing effects in mouse models of diabetes and ability to lower glucose in Phase 2 clinical trials. MSDC-0160 treatment of human islets increased AMPK activity and reduced mTOR activity. This was associated with the restoration of IGF-1-induced phosphorylation of Akt, GSK-3, and increased protein expression of Pdx1. Furthermore, MSDC-0160 in combination with IGF-1 and 8 mM glucose increased β-cell specific gene expression of insulin, pdx1, nkx6.1, and nkx2.2, and maintained insulin content without altering glucose-stimulated insulin secretion. Human islets were unable to simultaneously promote DNA synthesis and maintain the β-cell phenotype. Lithium-induced GSK-3 inhibition that promotes DNA synthesis blocked the ability of MSDC-0160 to maintain the β-cell phenotype. Conversely, MSDC-0160 prevented an increase in DNA synthesis by blocking β-catenin nuclear translocation. Due to the counteracting pathways involved in these processes, we employed a sequential ex vivo strategy to first induce human islet DNA synthesis, followed by MSDC-0160 to promote the β-cell phenotype and insulin content. This new generation PPARγ sparing insulin sensitizer may provide an initial tool for relieving inherent human islet insulin signaling pathway resistance that is necessary to preserve the β-cell phenotype during β-cell expansion for the treatment of diabetes.[1] |
| Molecular Formula |
C19H18N2O4S
|
|---|---|
| Molecular Weight |
370.4222
|
| Exact Mass |
370.098
|
| Elemental Analysis |
C, 61.61; H, 4.90; N, 7.56; O, 17.28; S, 8.65
|
| CAS # |
146062-49-9
|
| Related CAS # |
1628078-14-7 (R-isomer);146062-49-9 (racemic);1628078-13-6 (S-isomer);
|
| PubChem CID |
10429242
|
| Appearance |
Off-white to light yellow solid powder
|
| Density |
1.3±0.1 g/cm3
|
| Boiling Point |
623.8±55.0 °C at 760 mmHg
|
| Flash Point |
331.1±31.5 °C
|
| Vapour Pressure |
0.0±1.8 mmHg at 25°C
|
| Index of Refraction |
1.619
|
| LogP |
2.84
|
| Hydrogen Bond Donor Count |
1
|
| Hydrogen Bond Acceptor Count |
6
|
| Rotatable Bond Count |
7
|
| Heavy Atom Count |
26
|
| Complexity |
534
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
S1C(N([H])C(C1([H])C([H])([H])C1C([H])=C([H])C(=C([H])C=1[H])OC([H])([H])C(C1C([H])=C([H])C(=C([H])N=1)C([H])([H])C([H])([H])[H])=O)=O)=O
|
| InChi Key |
IRNJSRAGRIZIHD-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C19H18N2O4S/c1-2-12-5-8-15(20-10-12)16(22)11-25-14-6-3-13(4-7-14)9-17-18(23)21-19(24)26-17/h3-8,10,17H,2,9,11H2,1H3,(H,21,23,24)
|
| Chemical Name |
5-[[4-[2-(5-ethylpyridin-2-yl)-2-oxoethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione
|
| Synonyms |
Mitoglitazone; CAY10415; MSDC 0160; CAY 10415; MSDC-0160;CAY-10415; CAY 10415; CAY-10415; MSDC0160; CAY10415; 5-(4-(2-(5-ethylpyridin-2-yl)-2-oxoethoxy)benzyl)thiazolidine-2,4-dione; MSD-9;
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
DMSO: <1 mg/mL
Water: <1 mg/mL Ethanol: <1 mg/mL |
|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.75 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (6.75 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 2.5 mg/mL (6.75 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6996 mL | 13.4982 mL | 26.9964 mL | |
| 5 mM | 0.5399 mL | 2.6996 mL | 5.3993 mL | |
| 10 mM | 0.2700 mL | 1.3498 mL | 2.6996 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01374438 | Completed | Drug: MSDC-0160 Drug: Placebo |
Alzheimer's Disease | Metabolic Solutions Development Company |
July 2011 | Phase 2 |
| NCT00760578 | Completed | Drug: MSDC-0160 90 mg Drug: MSDC-0160 220 mg |
Type 2 Diabetes Mellitus | Metabolic Solutions Development Company |
September 2008 | Phase 2 |
| NCT01103414 | Completed | Drug: Mitoglitazone Drug: Pioglitazone |
Type 2 Diabetes | Metabolic Solutions Development Company |
September 2010 | Phase 2 |
 |
|---|
|
|