
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
Purity: ≥98%
LW6 is a hypoxia-inducible factor 1 (HIF) inhibitor which potently inhibits HIF-1α accumulation by degrading HIF-1α without affecting the HIF-1a mRNA levels during hypoxia. It inhibits HIF-1α transcription activity with IC50 of 2.64 μM in cell-based HRE-reporter gene assays. LW6 decreases HIF-1α protein expression without affecting HIF-1β expression. LW6 affects the stability of the HIF-1α protein. LW6 promotes the degradation of wild type HIF-1α, but not of a DM-HIF-1α with modifications of P402A and P564A, at hydroxylation sites in the oxygen-dependent degradation domain. LW6 did not affect the activity of prolyl hydroxylase (PHD), but induced the expression of von Hippel-Lindau (VHL), which interacts with prolyl-hydroxylated HIF-1alpha for proteasomal degradation.
| Targets |
HIF-1 (IC50 = 4.4 μM)
|
||
|---|---|---|---|
| ln Vitro |
The HIF-1α protein's stability is impacted by LW6. LW6 stimulates the hydroxylation sites in the oxygen-dependent degradation domain of wild type HIF-1α, but not that of a DM-HIF-1α with modifications of P402A and P564A. Von Hippel-Lindau (VHL) is induced by LW6 and interacts with prolyl-hydroxylated HIF-1α to facilitate proteasomal degradation. HIF-1α protein accumulation is not eliminated by VHL knockdown in the presence of LW6, suggesting that HIF-1α was degraded by LW6 through control of VHL expression[2]. LW6 is known to significantly increase the cellular accumulation of mitoxantrone, a substrate of BCRP, in MDCKII-BCRP cells that overexpress BCRP. BCRP expression is also down-regulated by LW6 at 0.1–10 µM concentrations[3]. At 20 µM, LW6 suppresses the expression of HIF 1α in A549 cells that have been hypoxic, without requiring the von Hippel Lindau protein. In addition to lowering the potential of the mitochondrial membrane, LW6 causes hypoxia-selective apoptosis[4].
|
||
| ln Vivo |
LW6 exhibits potent anti-tumor efficacy in vivo and reduces HIF-1α expression in frozen-tissue immunohistochemical staining in mice bearing xenografts of human colon cancer HCT116 cells[2].
LW6 inhibits growth of HCT116 cells in a xenograft tumor model[2] To evaluate the in vivo anti-tumor activity of LW6, we generated tumors in athymic nude mice by subcutaneous inoculation of the human cancer cell line HCT116. When the tumor volume reached ∼100 mm2, tumor-bearing mice were treated with LW6 (10 and 20 mg/kg) every day until the end of the study (Fig. 5 ). The administration of LW6 (20 mg/kg) significantly inhibited HCT116 tumor growth up to 53.6%, compared to that of the vehicle-treated control group (Fig. 5A). LW6-treated daily at a dose of 10 mg/kg produced 37.4% inhibition of tumor growth. These results suggest that LW6 is able to inhibit tumor growth dose-dependently. Topotecan, a clinical compound that suppresses both HIF-1α and HIF-2α protein accumulation, inhibited tumor growth by 73.7%. LW6 treatment did not cause significant weight loss or side effects, such as skin ulcers or other severe symptoms (Fig. 5B). To address whether the anti-tumor effect of LW6 was due to a decrease in HIF-1α, frozen sections of day 14 xenografts were processed for immunohistochemical staining with anti-HIF-1α antibody (Fig. 5C). The sections of tumors treated with either topotecan or LW6 showed low levels of HIF-1α, whereas high levels of HIF-1α were detected in untreated tumor tissue. This result further supports LW6, a HIF inhibitor, as a potential lead compound for cancer therapy. Furthermore, LW6 improved the oral exposure of methotrexate by twofold in rats[3]. |
||
| Enzyme Assay |
Reporter assay[2]
Inhibition of HIF-1α was assayed by a reporter assay using dual-luciferase reporter assay system, as previously described. HCT116 cells in 75–90% confluence were transiently co-transfected with pGL3-HRE-luciferase plasmid containing six copies of HREs from human VEGF genes and pRL-SV40 encoding firefly renilla luciferase and incubated for 24 h. Cells were treated with LW6 or 17-AAG for 16 h before report assay. Luciferase activity was integrated over a 10 second period and measured using a luminometer. The results were normalized to the activity of renilla luciferase. Data are presented as means ± SD. In vitro binding assay[2] The peptide substrates and GST-VBC proteins required for the binding assay were prepared as previously described. Fluorescence-labeled peptide substrate F-P564 (FITC-ACADLDLEALAPYIPADDDFQLR; final concentration 1 μM) was incubated with 0.2 μg/μl recombinant His6-PHD2 in NETN buffer containing 2 mM ascorbic acid, 5 mM 2-oxoglutarate, 100 μM FeCl2, and 5 μM N,N,N′,N′-tetrakis (2-pyridylmethyl) ethylenediamine (TPEN), with increasing concentrations of LW6 for 30 min at 25 °C. A reaction mixture containing DMSO alone without His6-PHD2 was included as a negative control, and another control contained F-HyP564 (a fluorescently labeled peptide with hydroxylation at P564). The reactions were terminated by heating for 1 min at 95 °C, followed by dilution to a final peptide concentration of 100 nM in EBC buffer (50 mM Tris, pH 8.0, 120 mM NaCl, 0.25% Nonidet P40) in the presence of 500 nM GST-VBC. Fluorescence polarization values were measured using a Luminescence Spectrometer LS50B. MDH2 Activity Assay[1] The activity of MDH2 was determined using a MDH2 activity assay kit according to the manufacturer’s instructions. Cells were lysed with RIPA buffer containing protease and phosphatase inhibitors. A 100 μL portion of proteins (100 μg/mL) was incubated for 3 h at room temperature in each well. After washing three times with washing buffer, 50 μL of drug diluted in base buffer was added to the well. This was preincubated for 1 h at room temperature and then for 3 h at 4 °C. The assay reagents (the final concentrations of malate and NAD+ were 5 and 1 mM, respectively) were added to the well, and the absorbance at 450 nm was measured at 30 and 60 min. In Vitro MDH2 Binding Assay[1] The recombinant human MDH2 (2 μg) was incubated with probes, and then photoaffinity labeling was performed by irradiation with 360 nm UV radiation for 30 min on ice. Click reactions of the probe and Cy3 azide were established with the Click-iT Protein Reaction Buffer Kit according to the manufacturer’s instructions. After the click reaction, proteins were precipitated with methanol/chloroform/water (60/15/40, v/v) and denatured by boiling for 5 min in a sample buffer. Proteins were separated by SDS-PAGE, and fluorescence was detected with a Typhoon 9410 imaging system. Kinetic Assay of MDH2[1] The enzyme activity of MDH2 was measured by oxaloacetate-dependent NADH oxidation assays, where the NADH concentration was determined by measuring absorbance at 340 nm. The reaction was performed in 100 mM potassium phosphate buffer (pH 7.4) with 0.25 nM His-MDH2, 600 μM oxaloacetic acid, and various concentrations of NADH (60, 75, 100, 150, and 300 μM). The Vmax and Km were determined from double-reciprocal Lineweaver–Burk plots using Sigmaplot 13.0, and a graph was plotted for the velocity against the concentration of NADH. |
||
| Cell Assay |
HRE-Luciferase Reporter Assay[1]
HCT116 cells expressing both a hypoxia response element (HRE)-dependent firefly luciferase reporter and a CMV-renilla luciferase reporter were cultured in DMEM supplemented with 5% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco). The cells (2 × 104 cells/0.1 mL/well) were seeded in a 96-well tissue culture plate for 20 h for subsequent experiments. The cells were incubated for 12 h with or without drugs in normoxic or hypoxic conditions (1% O2, 94% N2, and 5% CO2). The luciferase activities of cells were measured using a Dual-Luciferase Assay System with a Victor X Light luminescence reader. Immunoblot Analysis[1] Cells were washed with PBS, lysed with RIPA buffer (20 mM HEPES (pH 7.4), 1% Triton X-100, 10% glycerol, 1 mM EDTA, 5 mM sodium fluoride, 10 μg/mL phenylmethylsulfonylfluoride (PMSF), and 1 mM sodium vanadate) for 15 min at 4 °C, and centrifuged at 13 000 rpm for 15 min. Lysates were then boiled for 5 min in 5× sample buffer (50 mM Tris (pH 7.4), 4% sodium dodecyl sulfate (SDS), 10% glycerol, 4% 2-thioethanol, and 50 μg/mL bromophenol blue) at a ratio of 4:1. Protein samples were subjected to SDS-polyacrylamide gel electrophoresis (PAGE), transferred to PVDF membranes, and immunoblotted with anti-HIF-1α or anti-β-actin. Protein expression was visualized on Kodak Biomax X-ray film. Hypoxia-inducible factor HIF-1 is responsible for radiation resistance and poor prognosis in cancer therapy. As part of our drug discovery program, a novel HIF inhibitor, LW6, was identified as a small compound that inhibits the accumulation of HIF-1alpha. We found that LW6 decreased HIF-1alpha protein expression without affecting HIF-1beta expression. MG132, a proteasome inhibitor, protected HIF-1alpha from LW6-induced proteasomal degradation, indicating that LW6 affects the stability of the HIF-1alpha protein. We found that LW6 promoted the degradation of wild type HIF-1alpha, but not of a DM-HIF-1alpha with modifications of P402A and P564A, at hydroxylation sites in the oxygen-dependent degradation domain (ODDD). LW6 did not affect the activity of prolyl hydroxylase (PHD), but induced the expression of von Hippel-Lindau (VHL), which interacts with prolyl-hydroxylated HIF-1alpha for proteasomal degradation. In the presence of LW6, knockdown of VHL did not abolish HIF-1alpha protein accumulation, indicating that LW6 degraded HIF-1alpha via regulation of VHL expression. In mice carrying xenografts of human colon cancer HCT116 cells, LW6 demonstrated strong anti-tumor efficacy in vivo and caused a decrease in HIF-1alpha expression in frozen-tissue immunohistochemical staining. These data suggest that LW6 may be valuable in the development of a HIF-1alpha inhibitor for cancer treatment.[3] Effects of LW6 on the functional activity and gene expression of two major efflux transporters, BCRP and P-gp, were evaluated by using MDCKII cells overexpressing each transporter (MDCKII-BCRP and MDCKII-MDR1). Its effects on the cytotoxicity and pharmacokinetics of co-administered anticancer drugs were also evaluated in transfected cells.[4] |
||
| Animal Protocol |
|
||
| References |
|
||
| Additional Infomation |
A structure-activity relationship study of hypoxia inducible factor-1α inhibitor 3-aminobenzoic acid-based chemical probes, which were previously identified to bind to mitochondrial malate dehydrogenase 2, was performed to provide a better understanding of the pharmacological effects of LW6 and its relation to hypoxia inducible factor-1α (HIF-1α) and malate dehydrogenase 2 (MDH2). A variety of multifunctional probes including the benzophenone or the trifluoromethyl diazirine for photoaffinity labeling and click reaction were prepared and evaluated for their biological activity using a cell-based HRE-luciferase assay as well as a MDH2 assay in human colorectal cancer HCT116 cells. Among them, the diazirine probe 4a showed strong inhibitory activity against both HIF-1α and MDH2. Significantly, the inhibitory effect of the probes on HIF-1α activity was consistent with that of the MDH2 enzyme assay, which was further confirmed by the effect on in vitro binding activity to recombinant human MDH2, oxygen consumption, ATP production, and AMP activated protein kinase (AMPK) activation. Competitive binding modes of LW6 and probe 4a to MDH2 were also demonstrated.[1]
Purpose: The present study aimed to discover a new potent BCRP inhibitor overcoming multidrug resistance. Methods: Effects of LW6 on the functional activity and gene expression of two major efflux transporters, BCRP and P-gp, were evaluated by using MDCKII cells overexpressing each transporter (MDCKII-BCRP and MDCKII-MDR1). Its effects on the cytotoxicity and pharmacokinetics of co-administered anticancer drugs were also evaluated in transfected cells and rats, respectively. Results: In MDCKII-BCRP cells overexpressing BCRP, LW6 enhanced significantly (p < 0.05) the cellular accumulation of mitoxantrone, a BCRP substrate, and was more potent than Ko143, a well-known BCRP inhibitor. LW6 also down-regulated BCRP expression at concentrations of 0.1-10 µM. Furthermore, cells became more susceptible to the cytotoxicity of anticancer drugs in the presence of LW6. The CC50 values of mitoxantrone and doxorubicin were reduced by three- and tenfold, respectively, in MDCKII-BCRP cells, while LW6 did not affect the cytotoxicity of anticancer drugs in MDCKII-mock cells lacking BCRP transporter. Furthermore, LW6 improved the oral exposure of methotrexate by twofold in rats. In contrast to BCRP, LW6 had no inhibition effect on the functional activity and gene expression of P-gp. Conclusion: LW6 was newly identified as a potent BCRP inhibitor and could be useful to reduce the multidrug resistance of cancer cells via the inhibition of BCRP-mediated drug efflux as well as the down-regulation of BCRP expression.[2] |
| Molecular Formula |
C26H29NO5
|
|
|---|---|---|
| Molecular Weight |
435.51
|
|
| Exact Mass |
435.204
|
|
| Elemental Analysis |
C, 71.70; H, 6.71; N, 3.22; O, 18.37
|
|
| CAS # |
934593-90-5
|
|
| Related CAS # |
|
|
| PubChem CID |
16124726
|
|
| Appearance |
white solid powder
|
|
| Density |
1.3±0.1 g/cm3
|
|
| Boiling Point |
647.4±55.0 °C at 760 mmHg
|
|
| Flash Point |
345.3±31.5 °C
|
|
| Vapour Pressure |
0.0±2.0 mmHg at 25°C
|
|
| Index of Refraction |
1.637
|
|
| LogP |
6.49
|
|
| Hydrogen Bond Donor Count |
2
|
|
| Hydrogen Bond Acceptor Count |
5
|
|
| Rotatable Bond Count |
7
|
|
| Heavy Atom Count |
32
|
|
| Complexity |
659
|
|
| Defined Atom Stereocenter Count |
0
|
|
| SMILES |
O=C(C1C=C(NC(COC2C=CC(C34CC5CC(C3)CC(C5)C4)=CC=2)=O)C(O)=CC=1)OC
|
|
| InChi Key |
BJRPPNOJYFZSLY-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C26H29NO5/c1-31-25(30)19-2-7-23(28)22(11-19)27-24(29)15-32-21-5-3-20(4-6-21)26-12-16-8-17(13-26)10-18(9-16)14-26/h2-7,11,16-18,28H,8-10,12-15H2,1H3,(H,27,29)
|
|
| Chemical Name |
methyl 3-[[2-[4-(1-adamantyl)phenoxy]acetyl]amino]-4-hydroxybenzoate
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: 2.5 mg/mL (5.74 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with sonication.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (5.74 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2962 mL | 11.4808 mL | 22.9616 mL | |
| 5 mM | 0.4592 mL | 2.2962 mL | 4.5923 mL | |
| 10 mM | 0.2296 mL | 1.1481 mL | 2.2962 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
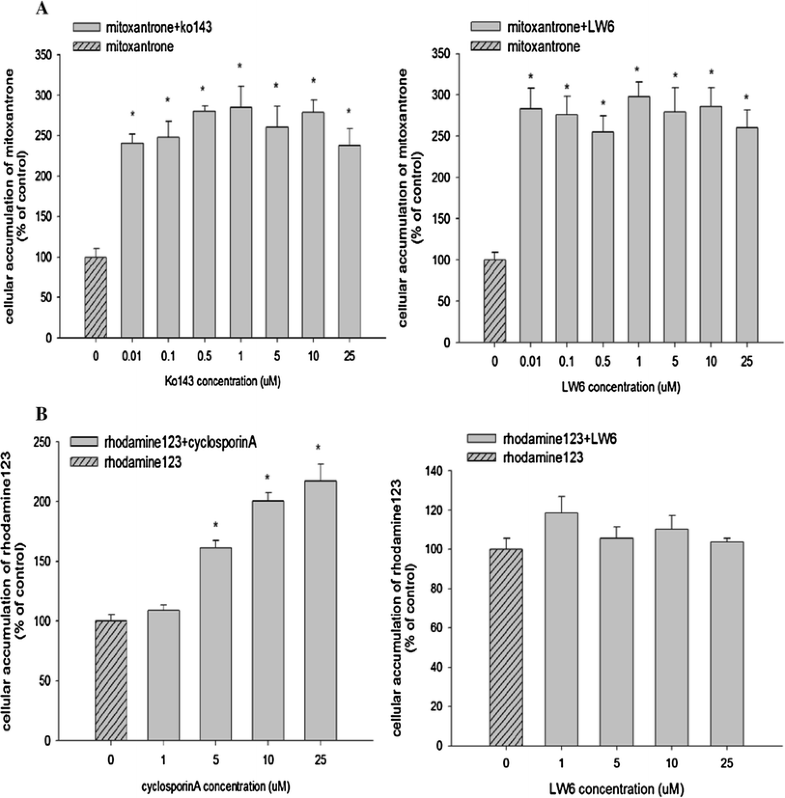 Inhibition effect of LW6 on the cellular accumulation of mitoxantrone in MDCKII-BCRP cells (a) and rhodamine-123 in MDCKII-MDR1 cells (b) (mean±SD,n=6). *pCancer Chemother Pharmacol.2016 Oct;78(4):735-44. |
|---|
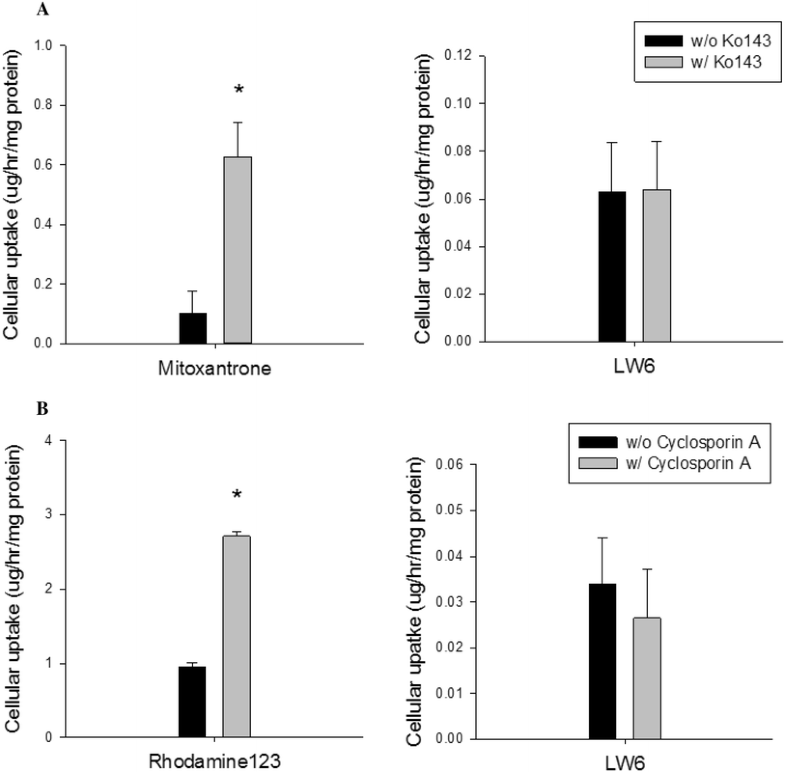 Cellular uptake studies of LW6 in MDCKII-BCRP cells (a) and MDCKII-MDR1 cells (b) (mean±SD,n=6).Cancer Chemother Pharmacol.2016 Oct;78(4):735-44. |
Effect of LW6 on the expression of BCRP.Cancer Chemother Pharmacol.2016 Oct;78(4):735-44. |
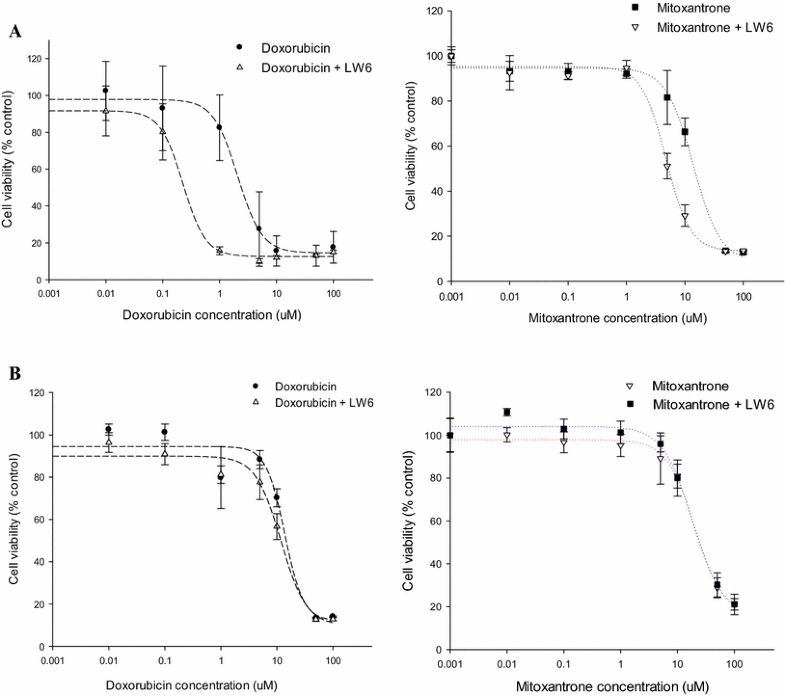 Cytotoxicity of mitoxantrone and doxorubicin with/without LW6 in MDCKII-BCRP.Cancer Chemother Pharmacol.2016 Oct;78(4):735-44. |
|---|
Plasma concentration–time profiles of methotrexate after an oral administration of methotrexate (10mg/kg) in the presence and absence of LW6 (10mg/kg) in rats (mean±SD,n=5).Cancer Chemother Pharmacol.2016 Oct;78(4):735-44. |