
| Size | Price | Stock | Qty |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g | |||
| Other Sizes |
Purity: ≥98%
Erismodegib (formerly LDE-225; NVP LDE-225; LDE225; NVP-LDE22; Sonidegib; trade name of Odomzo) is an orally bioavailable small-molecule antagonist of the Smoothened (Smo) in Hedgehog signaling pathway with potential antitumor activity. It suppresses Hedgehog (Hh) signaling with an IC50 of 1.3 nM for mice and 2.5 nM for humans in cell-free assays. LDE225 (NVP-LDE225, Erismodegib, Sonidegib), an anticancer drug that has been approved, binds specifically to the cell surface receptor Smo that carries the Hedgehog (Hh) ligand. This suppresses the Hedgehog signaling pathway, which restricts the growth of tumor cells that have an aberrantly activated Hedgehog pathway. For the treatment of basal cell carcinoma, the FDA approved this anticancer medication in 2015.
| Targets |
mSmo ( IC50 = 1.3 nM ); hSmo ( IC50 = 2.5 nM )
|
|
|---|---|---|
| ln Vitro |
|
|
| ln Vivo |
|
|
| Enzyme Assay |
Fluorescence binding assays using BODIPY-cyclopamine [1]
Fluorescence binding assays using BODIPY FL or BODIPY® 558/568 labeled binding assays were conducted as described. Briefly, binding assays were conducted in 384-well plates using fixed CHO cells stably expressing mouse or human Smo. Cells were fixed with 4% paraformaldehyde for 15 min at room temperature, washed, covered in PBS buffer containing 0.5% fetal bovine serum, and incubated with fluorescence labeled BODIPY-cyclopamine (20 nM) and the test compounds [e.g. Sonidegib (Erismodegib; LDE225; NVP-LDE225)] for 4 h at 37 °C. The treated cells then were washed with PBS, stained with Hoechst 33258, and analyzed by ImageXpress® Ultra imaging system. TM3-Gli-Luc reporter gene assay [1] Test compounds [e.g. Sonidegib (Erismodegib; LDE225; NVP-LDE225)] were prepared for assay by serial dilution in DMSO and then added to empty assay plates. TM3Hh12 cells (TM3 cells containing Hh-responsive reporter gene construct pTA8xGli-Luc) were cultured in F12 Ham’s/DMEM (1:1) containing 5% horse serum, 2.5% fetal bovine serum (FBS), and 15 mM HEPES, pH 7.3. Cells were harvested by trypsin treatment, resuspended in F12 Ham’s/DMEM (1:1) containing 5% horse serum and 15 mM HEPES, pH 7.3, added to assay plates, and incubated with test compounds for approximately 30 min at 37 °C in 5% CO2. Then 1 or 25 nM Ag1.5 was added to assay plates and incubated at 37 °C in the presence of 5% CO2. After 48 h, either Bright-Glo (Promega E2650) or MTS reagent was added to the assay plates and luminescence or absorbance at 492 nm was determined. IC50 values, defined as the inflection point of the logistic curve, were determined by nonlinear regression of the Gli-driven luciferase luminescence or absorbance signal from MTS assay vs log10 (concentration) of test compounds using the R statistical software package. [1] LLDE225 blocks the TM3 luciferized cell line with 0.6 nM and 8 nM of Hh agonist Ag1.5 present, respectively. |
|
| Cell Assay |
Proliferation/apoptosis/cell cycle analysis[2]
CD34+ CP-CML cells were seeded in SFM alone ± Sonidegib (Erismodegib; LDE225; NVP-LDE225) ± nilotinib and cultured for 24–72 h prior to assessment. Proliferation was measured using colorimetric assessment of BrDU incorporation. Proportion of viable cells versus those in early and late apoptosis was assessed by flow cytometry using annexin V–FITC and 7-amino-actinomycin D (7-AAD, Via-Probe solution) according to the manufacturer’s instructions. Cell cycle status was assessed as previously described using Ki67 (FITC) expression and 7-AAD incorporation55. CFC assay/re-plating assay[2] CD34+ CP-CML cells were seeded in SFM ± Sonidegib (Erismodegib; LDE225; NVP-LDE225) ± nilotinib and cultured for 72 h then washed three times, inoculated at a concentration of 4 × 103/ml into methylcellulose supplemented with growth factors and cultured in duplicate for 14d prior to colony assessment. Following assessment, at least 20 colonies (granulocyte-erythroid-megakaryocyte-macrophage [GEMM] or granulocyte macrophage [GM]) colonies were plucked from each experimental arm and serially re-dispersed in Methocult with secondary and tertiary colony formation assessed after 7d intervals. LTC-IC assay[2] Primary CD34+ normal and CP-CML cells were cultured in SFM ±Sonidegib (Erismodegib; LDE225; NVP-LDE225) ± nilotinib for 72 h. Following this, they were thoroughly washed and inoculated into pre-prepared long term cultures comprising a stromal feeder layer (a 1:1 mix of irradiated (80 Gy) SL/SL and M210B4 murine fibroblasts) in long term myeloid culture medium (MyeloCult supplemented with hydrocortisone) as previously described35. These cultures were maintained for 5 weeks with 50% media changes performed weekly. Following this, the contents of the wells were harvested and cells counted prior to seeding into Methocult to perform CFC assays as described above. Long term stromal co-culture[2] CD34+ CP-CML cells were inoculated directly into pre-prepared stromal co-cultures, as described above, in the presence of Sonidegib (Erismodegib; LDE225; NVP-LDE225) ± nilotinib. Cultures were maintained for 5 weeks with 80% media changes and addition of fresh drug weekly. Co-cultures were examined weekly by microscopy to ensure that the stromal layer remained morphologically normal and adherent. After 5 weeks, CFC assays were performed as described. Prior to assessment, CD34+ CP-CML cells are cultured for 24-72 hours in SFM alone±Sonidegib±Nilotinib. BrDU incorporation colorimetric assessment is used to quantify proliferation. Utilizing annexin V-FITC and 7-amino-actinomycin D (7-AAD, Via-Probe solution), flow cytometry is used to determine the ratio of viable cells to those in early and late apoptosis. Ki67 (FITC) expression and 7-AAD incorporation are used to determine the cell cycle status. |
|
| Animal Protocol |
|
|
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
Sonidegib is rapidly absorbed in the fasted state with peak concentrations occurring 2-4 hours after administration. (2) However, the total absorption of Sonidegib is low (roughly 6-7%). (1) Around 70% of Sonidegib is eliminated in the feces, while 30% is eliminated in the urine. (2) Estimated volume of distribution = 9166 L (2) Metabolism / Metabolites Sonidegib is primarily metabolized via oxidation and amide hydrolysis. (1) The enzyme responsible for the majority of metabolism is the cytochrome P450 (CYP) 3A4 enzyme. (2) Biological Half-Life Half-life ~ 28 days (2) |
|
| Toxicity/Toxicokinetics |
Hepatotoxicity
Most clinical trials of sonidegib included few patients and rates of liver tests abnormalities were often not reported. In isolated trials, serum ALT elevations were reported in 15% to 27% of patients and to rise above 5 times the upper limit of normal (ULN) in 1% to 6%. Rates of serum enzyme elevations were greater with higher doses, and all were apparently transient and resolved either spontaneously or with dose reductions or discontinuation. In these trials, there were no cases of clinically apparent liver injury, hepatitis with jaundice or death from liver failure. The product label for sonidegib mentions serum enzyme elevations as a possible adverse event, but does not mention liver injury with jaundice or hepatic failure. Since its approval and more widespread use, there have been no published cases of hepatotoxicity attributed to sonidegib, but it is an uncommonly used antineoplastic agent. Serum enzyme elevations were also rare with the initial hedgehog inhibitor, vismodegib, which has been implicated in causing at least one case of acute, self-limited cholestatic hepatitis (Case 1 in Vismodegib). Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of sonidegib during breastfeeding. Because sonidegib is 97% bound to plasma proteins, the amount in milk is likely to be low. However, its half-life is about 28 days and it might accumulate in the infant. The manufacturer recommends that breastfeeding be discontinued during sonidegib therapy. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Sonidegib is over 97% bound to plasma proteins, and binding is independent of concentration. (2) |
|
| References | ||
| Additional Infomation |
Pharmacodynamics
Sonidegib has been shown to inhibit a transmembrane protein called SMO which plays a role in Hh signal transduction. This has resulted in inhibition of Hh signaling as well as antitumour activity in various animal models. In a transgenic mouse model of islet cell neoplasms, tumour volume was reduce by 95% in mice treated with sonidegib when compared with untreated mice. (2) |
| Molecular Formula |
C26H26F3N3O3
|
|---|---|
| Molecular Weight |
485.5
|
| Exact Mass |
485.192
|
| Elemental Analysis |
C, 64.32; H, 5.40; F, 11.74; N, 8.66; O, 9.89
|
| CAS # |
956697-53-3
|
| Related CAS # |
Sonidegib diphosphate; 1218778-77-8
|
| PubChem CID |
24775005
|
| Appearance |
White to light yellow solid powder
|
| Density |
1.3±0.1 g/cm3
|
| Boiling Point |
544.5±50.0 °C at 760 mmHg
|
| Flash Point |
283.1±30.1 °C
|
| Vapour Pressure |
0.0±1.5 mmHg at 25°C
|
| Index of Refraction |
1.569
|
| LogP |
5.43
|
| Hydrogen Bond Donor Count |
1
|
| Hydrogen Bond Acceptor Count |
8
|
| Rotatable Bond Count |
5
|
| Heavy Atom Count |
35
|
| Complexity |
691
|
| Defined Atom Stereocenter Count |
2
|
| SMILES |
CC1C(C(=O)NC2C=NC(N3C[C@H](C)O[C@H](C)C3)=CC=2)=CC=CC=1C1C=CC(OC(F)(F)F)=CC=1
|
| InChi Key |
VZZJRYRQSPEMTK-CALCHBBNSA-N
|
| InChi Code |
InChI=1S/C26H26F3N3O3/c1-16-14-32(15-17(2)34-16)24-12-9-20(13-30-24)31-25(33)23-6-4-5-22(18(23)3)19-7-10-21(11-8-19)35-26(27,28)29/h4-13,16-17H,14-15H2,1-3H3,(H,31,33)/t16-,17+
|
| Chemical Name |
N-[6-[(2S,6R)-2,6-dimethylmorpholin-4-yl]pyridin-3-yl]-2-methyl-3-[4-(trifluoromethoxy)phenyl]benzamide
|
| Synonyms |
Sonidegib; LDE 225; NVP-LDE225; LDE-225; NVP LDE-225; LDE225; NVP LDE225; Erismodegib; trade name of Odomzo
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (5.15 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: 2.5 mg/mL (5.15 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 2.5 mg/mL (5.15 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 4: 2% DMSO+corn oil: 10 mg/mL Solubility in Formulation 5: 2 mg/mL (4.12 mM) in 75% PEG 300 25% (5% dextrose in water) (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0597 mL | 10.2987 mL | 20.5973 mL | |
| 5 mM | 0.4119 mL | 2.0597 mL | 4.1195 mL | |
| 10 mM | 0.2060 mL | 1.0299 mL | 2.0597 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
Oral Hedgehog Inhibitors in the Treatment of Basal Cell Carcinoma in the Netherlands: a Prospective Registration Study
CTID: NCT05463757
Phase: Status: Recruiting
Date: 2024-05-22
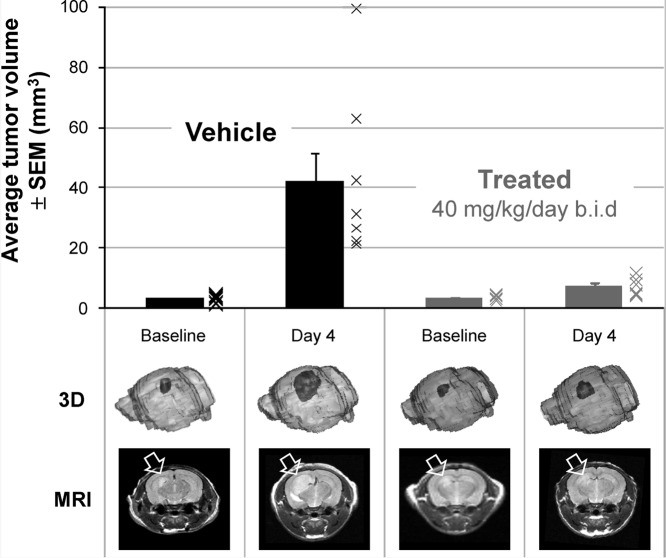 Antitumor activity in an orthotopic Ptch+/−p53−/−medulloblastoma allograft model in nude mice upon treatment with5mdiphosphate salt dosed at 40 mg/kg/day po bid or vehicle at equal dose volume.ACS Med Chem Lett. 2010 Jun 10; 1(3): 130–134. |
|---|
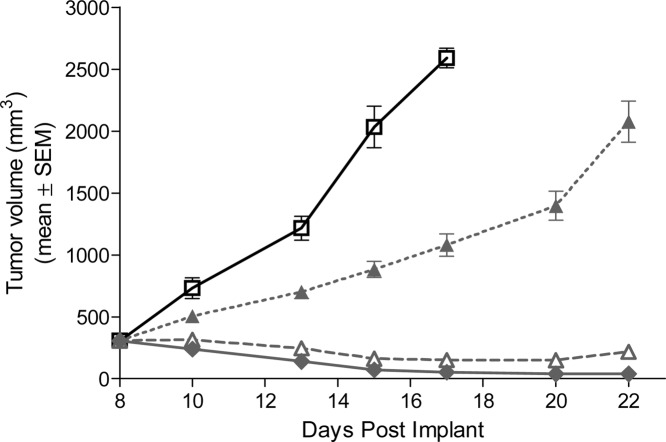 Antitumor activity upon treatment with5mdiphosphate salt or vehicle in a Ptch+/−p53−/− medulloblastoma subcutaneous allograft model in nude mice.ACS Med Chem Lett. 2010 Jun 10; 1(3): 130–134. |
Gli1 mRNA inhibition (open circle), tumor PK (filled squares), and plasma PK (filled triangles) in Ptch+/−p53−/−medulloblastoma model after treatment with5m (Sonidegib, or erismodegib, LDE225, NVP-LDE225).ACS Med Chem Lett. 2010 Jun 10; 1(3): 130–134. |