
| Size | Price | Stock | Qty |
|---|---|---|---|
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| Other Sizes |
|
Purity: ≥98%
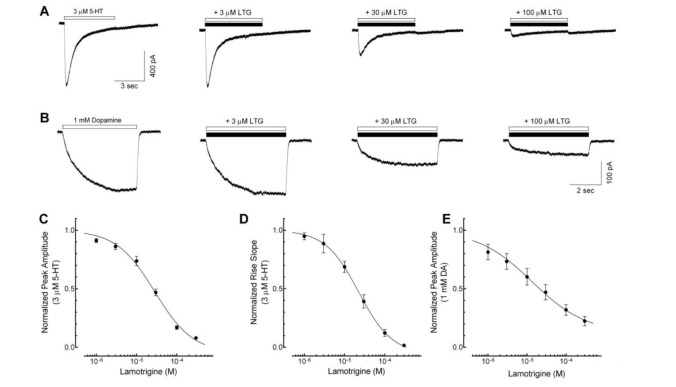
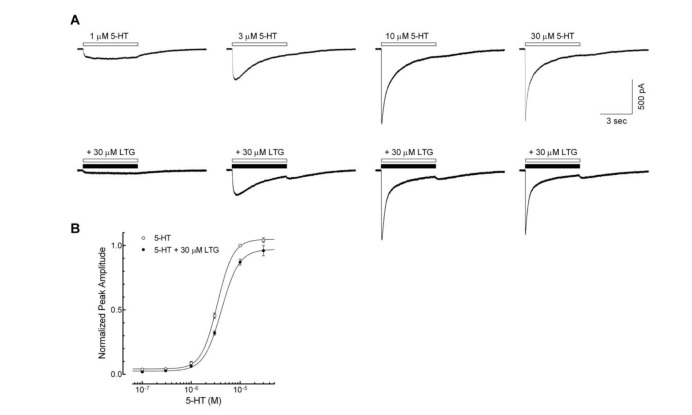
Lamotrigine (Lamictal, BW 430C; BW430C; Crisomet, Lamictin, Lamitor), an approved anti-convulsant drug used in the treatment of epilepsy and bipolar disorder, is an inhibitor of 5-HT with IC50 of 240 μM and 474 μM in human platelets and rat brain synaptosomes. Moreover, lamotrigine blocks sodium channels.
| Targets |
Sodium channel; 5-HT (human platelets) ( IC50 = 240 μM ); 5-HT (rat brain synaptosomes) ( IC50 = 474 μM )
|
||
|---|---|---|---|
| ln Vitro |
|
||
| ln Vivo |
|
||
| Animal Protocol |
|
||
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
Lamotrigine is rapidly and entirely absorbed with minimal first-pass metabolism effects, with a bioavailability estimated at 98%. Cmax is reached in the range of 1.4 to 4.8 hours post-dose, but this depends on the dose administered, concomitant medications, and epileptic status. The rate and extent of lamictal absorption is considered equivalent between the compressed tablet form taken with water to that of the chewable dispersible tablets, taken with or without water. Lamotrigine is excreted in both the urine and feces. Following oral administration of 240 mg radiolabelled lamotrigine, about 94% of total drug and its metabolites administered is recovered in the urine and 2% is recovered in the feces. One pharmacokinetic study recovered 43 to 87% of a lamotrigine dose in the urine mainly as glucuronidated metabolites. 2-N-glucuronide is mainly excreted in the urine. The mean apparent volume of distribution (Vd/F) of lamotrigine following oral administration ranges from 0.9 to 1.3 L/kg and is independent of dose administered. Lamotrigine accumulated in the kidney of the male rat, and likely behaves in a similar fashion in humans. Lamotrigine also binds to tissues containing melanin, such as the eyes and pigmented skin. The mean apparent plasma clearance (Cl/F) ranges from 0.18 to 1.21 mL/min/kg. The values vary depending on dosing regimen, concomitant antiepileptic medications, and disease state of the individual. In one study, healthy volunteers on lamictal monotherapy showed a clearance of about 0.44 mL/min/kg after a single dose. /MILK/ Lamotrigine is distributed into milk. Because of the potential for serious adverse reactions to lamotrigine in nursing infants, a decision should be made whether to discontinue nursing or the drug, taking into account the importance of the drug to the woman. /MILK/ To investigate the pharmacokinetics of lamotrigine (LTG) during delivery, during the neonatal period, and lactation. High-performance liquid chromatography was used to determine plasma and milk levels of LTG in nine pregnant women with epilepsy treated with LTG, and plasma levels in their 10 infants. Samples were obtained at delivery, the first 3 days postpartum, and at breast-feeding 2-3 weeks after delivery. At delivery, maternal plasma LTG concentrations were similar to those from the umbilical cord, indicating extensive placental transfer of LTG. There was a slow decline in the LTG plasma concentration in the newborn. At 72 hr postpartum, median LTG plasma levels in the infants were 75% of the cord plasma levels (range, 50-100%). The median milk/maternal plasma concentration ratio was 0.61 (range, 0.47-0.77) 2-3 weeks after delivery, and the nursed infants maintained LTG plasma concentrations of approximately 30% (median, range 23-50%) of the mother's plasma levels. Maternal plasma LTG concentrations increased significantly during the first 2 weeks after parturition, the median increase in plasma concentration/dose ratio being 170%. Lamotrigine binds to melanin-containing ocular tissue in pigmented rats and cynomolgus monkeys, but evidence of this manifestation has not been reported in humans ... prolonged administration of the drug could potentially result in its accumulation and possible toxic effects in melanin-rich tissues, including those of the eye, and that clinicians should be aware of possible adverse ophthalmologic effects occurring as a result of binding of the drug to melanin. To determine the relative bioavailability of lamotrigine (LTG) chewable dispersible tablets after rectal administration. Two-period, crossover study with a 2-week washout between dosing periods. Twelve healthy adult volunteers. One hundred milligrams of a LTG chewable dispersible tablet was administered by oral and rectal routes. Plasma samples were collected before and up to 120 hours after drug administration. The samples were analyzed for LTG by high-performance liquid chromatography, and the relative bioavailability was determined. Drug concentrations were lower after rectal than after oral administration. The relative bioavailability (F = AUC(rectal)/AUC(oral)) was 0.52 +/- 0.23 (SD). Drug prepared from LTG chewable dispersible tablets is absorbed rectally, although not to the same extent as when given orally. Rectal administration of suspension of these tablets can be an acceptable route of administration. For more Absorption, Distribution and Excretion (Complete) data for LAMOTRIGINE (9 total), please visit the HSDB record page. Metabolism / Metabolites Lamotrigine is mainly glucuronidated, forming 2-N-glucuronide conjugate, a pharmacologically inactive metabolite. The total radioactivity detected after a 240mg radiolabeled dose of lamotrigine during clinical trials were as follows: lamotrigine as unchanged drug(10%), a 2-N-glucuronide (76%), a 5-N-glucuronide (10%), a 2-N-methyl metabolite (0.14%), as well as various other minor metabolites (4%). The metabolites of [(14)C]lamotrigine (78 micromol/kg, iv) in adult male Wistar rats were characterized with particular reference to thioether derivatives of an epoxide intermediate. Biliary recovery of radioactivity from anesthetized and cannulated animals was 7.3 +/- 3.0% (mean +/- SD, n = 4) of the dose over 4 hr; 5.5 +/- 0.5% was recovered in bladder urine after 4 hr. Bile contained [(14)C]lamotrigine (1.4 +/- 0.3%), a glutathione adduct of [(14)C]dihydrohydroxylamotrigine (1.8 +/- 0.3%), i.e., an adduct of an arene oxide, and the glutathione (1.5 +/- 0.7%), cysteinylglycine (1.9 +/- 0.5%), and N-acetylcysteine (0.4 +/- 0.2%) adducts of [(14)C]lamotrigine. Formation of the thioether metabolites was partially blocked by the cytochrome P450 inhibitor, ketoconazole. Urine contained [(14)C]lamotrigine (4.5 +/- 0.5%) and [(14)C]lamotrigine N-oxide (0.9 +/- 0.2%). The radiolabeled material in skin (15.6 +/- 1.4%) was almost entirely [(14)C]lamotrigine. ... Lamotrigine is metabolized predominantly by glucuronic acid conjugation. The major metabolite is an inactive 2-N-glucuronide conjugate. Exretion occur in the urine and the feces with unchanged lamotrigine (10%), the 2-N-glucuronide (76%), a 5-N-glucuronide (10%), a 2-N-methyl metabolite (0.14%), and other unidentified minor metabolites (4%). (A308) Half Life: 25 +/- 10 hours (healthy individuals); 42.9 hours (chronic renal failure) Biological Half-Life The average elimination half-life of lamotrigine ranges from approximately 14-59 hours. The value is dependent on the dose administered, concomitant drug therapy, as well as disease status. One pharmacokinetic study revealed a half-life of 22.8 to 37.4 hours in healthy volunteers. It also reported that enzyme-inducing antiepileptic drugs such as pheobarbital, phenytoin, or carbamazepine decrease the half-life of lamotrigine. On the other hand, valproic acid increases the half-life of lamotrigine (in the range of 48-59 hours). /Investigators/ describe the findings in a patient following the deliberate ingestion of a large amount of lamotrigine (stated 4.5 g, absorbed estimated 2.9 g) ... Peak measured concentration of lamotrigine was 35.8 mg/L and half-life 19.5 hours, suggesting linear kinetics in overdose. The plasma half-life of a single dose is 24 to 35 hr. Administration of phenytoin, carbamazepine, phenobarbital, or primidone reduces the half-life of lamotrigine to approximately 15 hr and reduces plasma concentrations of lamotrigine. |
||
| Toxicity/Toxicokinetics |
Toxicity Summary
IDENTIFICATION AND USE: Lamotrigine is a white to pale cream-colored powder. Lamotrigine is an anticonvulsant medication that also has utility in the treatment of bipolar disorder. HUMAN EXPOSURE AND TOXICITY: Lamotrigine has been associated with many side effects, including rashes that can progress to Stevens-Johnson syndrome or toxic epidermal necrolysis. It has also been associated with the development of motor tics, most commonly in the head, neck, and shoulders. Cases of life-threatening rashes associated with lamotrigine almost always have occurred within 2-8 weeks of treatment initiation; however, severe rashes rarely have presented following prolonged treatment (e.g., 6 months). Lamotrigine-associated rashes do not appear to have distinguishing features. Because it is not possible to distinguish benign rashes from those that may become severe and/or life-threatening, lamotrigine generally should be discontinued at the first sign of rash (unless the rash is known not to be drug related). However, a rash may become life-threatening or permanently disabling or disfiguring despite discontinuance of the drug. Discontinuance of lamotrigine because of rash was required in 3% of adults receiving the drug as adjunctive therapy and 4.5% of adults receiving the drug as monotherapy in controlled clinical trials; 4.4% of pediatric patients receiving lamotrigine in controlled clinical trials discontinued the drug because of the development of rash. Multiorgan failure and various degrees of hepatic failure, in some cases fatal, have been reported rarely with lamotrigine as adjunctive therapy. The possibility of such potentially fatal adverse effects should be considered in patients who exhibit signs and symptoms associated with multiorgan and/or hepatic impairment following initiation of lamotrigine as adjunctive therapy. During the premarketing development of lamotrigine, 20 sudden and unexplained deaths were reported among a cohort of 4700 patients with epilepsy receiving adjunctive therapy with the drug (5747 patient-years of exposure). Although the rate of these deaths exceeds that expected to occur in a healthy (nonepileptic) population matched for age and gender, this rate was similar to that occurring in a similar population of epileptic patients receiving a chemically unrelated anticonvulsant agent. Among 414 first-trimester exposures to lamotrigine monotherapy, 12 outcomes with major birth defects were reported. Among the 88 first-trimester exposures to lamotrigine polytherapy including valproate, 11 outcomes with major birth defects were reported. Among 182 first-trimester exposures to lamotrigine polytherapy excluding valproate, 5 outcomes with major birth defects were reported. No distinctive pattern of major birth defects was apparent among the offspring exposed to lamotrigine monotherapy or polytherapy. The risk of all major birth defects after first-trimester exposure to lamotrigine monotherapy (2.9%) was similar to that in the general population and in other registries enrolling women exposed to antiepileptic monotherapy (3.3% to 4.5%). Lamotrigine also did not increase the incidence of structural or numerical chromosomal abnormalities in the in vitro human lymphocyte assay. ANIMAL STUDIES: In animal studies, no evidence of carcinogenicity was seen following oral administration of lamotrigine for up to 2 years at maximum tolerated doses (30 mg/kg of body weight per day in mice and 10 to 15 mg/kg per day in rats). Lamotrigine administered i.p. at high doses can induce intrauterine growth retardation and at low multiple doses causes a dose-dependent increase in embryonic resorption, craniofacial and caudal malformations as well as maternal toxicity in the mouse. A study of the teratogenic activity of lamotrigine was carried out in the brain of fetuses of rats who had received the drug. Results showed that fetuses of the experimental group had reduced body weight at birth, increased volume and diameter of the cerebral structure, increased density of the subcortical layer, and ventricle dilatation. A behavioral teratology study was conducted in rats dosed during the period of organogenesis. At day 21 postpartum, offspring of dams receiving 5 mg/kg per day or higher displayed a significantly longer latent period for open field exploration and a lower frequency of rearing. In a swimming maze test performed on days 39 to 44 postpartum, time to completion was increased in offspring of dams receiving 25 mg/kg per day. No evidence of mutagenicity was demonstrated by lamotrigine in vitro in the Ames Salmonella microbial mutagen test or the mammalian mouse lymphoma assay. Lamotrigine also did not increase the incidence of structural or numerical chromosomal abnormalities in the in vivo rat bone marrow assay. One proposed mechanism of action of Lamotrigine, the relevance of which remains to be established in humans, involves an effect on sodium channels. in vitro pharmacological studies suggest that lamotrigine inhibits voltage-sensitive sodium channels and/or calcium channels, thereby stabilizing neuronal membranes and consequently modulating presynaptic transmitter release of excitatory amino acids (e.g., glutamate and aspartate). Studies on lamotrigine show binding to sodium channels similar to local anesthetics. Toxicity Data LD50: 250 (mg/kg) (mice) LD50: 250 (mg/kg) (rat) LD50> 640 (mg/kg) (oral, rat) (Sawyer) LD50> 640 (mg/kg) (oral, mice) (Sawyer) Interactions Rash, including serious and potentially life-threatening rash, appears to be more likely to occur in patients receiving concomitant valproic acid. Valproic acid can decrease clearance and increase plasma concentrations of lamotrigine more than twofold; exceeding the recommended reduced initial dosage of lamotrigine or the subsequent recommended schedule for escalation of lamotrigine dosage, particularly in patients receiving valproic acid, may increase the incidence of rash, including serious rash, in lamotrigine-treated patients. In clinical trials, 1% of adults and 1.2% of children receiving a drug regimen including immediate-release lamotrigine concomitantly with valproic acid experienced a rash requiring hospitalization, while 0.16% of adults and 0.6% of children receiving a drug regimen of lamotrigine without valproic acid were hospitalized because of rash. The concomitant use of valproic acid and/or hepatic enzyme-inducing anticonvulsant drugs (e.g., phenobarbital, primidone, carbamazepine, phenytoin) can increase or decrease the metabolism and elimination of lamotrigine, requiring dosage adjustments to maintain efficacy and/or avoid toxicity. Addition of valproic acid to lamotrigine therapy reduces lamotrigine clearance and increases steady-state plasma lamotrigine concentrations by slightly more than 50% whether or not hepatic enzyme-inducing anticonvulsant drugs are given concomitantly. Conversely, steady-state plasma concentrations of lamotrigine are decreased by about 40% when phenobarbital, primidone, or carbamazepine is added to lamotrigine therapy and by about 45-54% when phenytoin is added to lamotrigine therapy; the magnitude of the effect with phenytoin is dependent on the total daily dosage of phenytoin (from 100-400 mg daily). Discontinuance of an enzyme-inducing anticonvulsant drug can be expected to increase, and discontinuance of valproic acid can be expected to decrease, the elimination half-life and plasma concentrations of lamotrigine. Although the manufacturers state that a therapeutic plasma concentration range has not been established for lamotrigine and that dosage should be based on therapeutic response, the change in plasma lamotrigine concentrations resulting from addition or discontinuance of enzyme-inducing anticonvulsant drugs or valproic acid should be considered when these drugs are added to or withdrawn from an existing anticonvulsant drug regimen that includes lamotrigine. Addition of lamotrigine to existing therapy with phenytoin or carbamazepine generally does not appreciably alter the steady-state plasma concentrations of these concomitantly administered drugs. Addition of lamotrigine to carbamazepine therapy reportedly has resulted in increased plasma concentrations of a pharmacologically active metabolite of carbamazepine (carbamazepine-10,11-epoxide) and an increased incidence of some adverse effects (e.g., dizziness, headache, diplopia, blurred vision, ataxia, nausea, nystagmus). However, elevations in carbamazepine-10,11-epoxide plasma concentrations and/or increased toxicity have not been consistently observed with concomitant administration of lamotrigine and carbamazepine, and the mechanism of the interaction between these drugs remains unclear. Addition of lamotrigine to valproic acid therapy in healthy individuals resulted in a 25% reduction in trough steady-state plasma concentrations of valproic acid over a 3-week period, followed by stabilization of these concentrations. For more Interactions (Complete) data for LAMOTRIGINE (11 total), please visit the HSDB record page. |
||
| References | |||
| Additional Infomation |
Therapeutic Uses
Anticonvulsants; Calcium Channel Blockers; Excitatory Amino Acid Antagonists; Voltage-Gated Sodium Channel Blockers /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Lamotrigine is included in the database. Lamictal is indicated as adjunctive therapy for the following seizure types in patients aged 2 years and older: partial-onset seizures, primary generalized tonic-clonic (PGTC) seizures, generalized seizures of Lennox-Gastaut syndrome. /Included in the US product label/ Lamcital is indicated for conversion to monotherapy in adults (aged 16 years and older) with partial-onset seizures who are receiving treatment with carbamazepine, phenytoin, phenobarbital, primidone, or valproate as the single antiepileptic drug (AED). /Included in US product label/ For more Therapeutic Uses (Complete) data for LAMOTRIGINE (6 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ WARNING: SERIOUS SKIN RASHES. Lamictal can cause serious rashes requiring hospitalization and discontinuation of treatment. The incidence of these rashes, which have included Stevens-Johnson syndrome, is approximately 0.3% to 0.8% in pediatric patients (aged 2 to 17 years) and 0.08% to 0.3% in adults receiving Lamictal. One rash-related death was reported in a prospectively followed cohort of 1,983 pediatric patients (aged 2 to 16 years) with epilepsy taking Lamictal as adjunctive therapy. In worldwide postmarketing experience, rare cases of toxic epidermal necrolysis and/or rash-related death have been reported in adult and pediatric patients, but their numbers are too few to permit a precise estimate of the rate. Other than age, there are as yet no factors identified that are known to predict the risk of occurrence or the severity of rash caused by Lamictal. There are suggestions, yet to be proven, that the risk of rash may also be increased by (1) coadministration of Lamictal with valproate (includes valproic acid and divalproex sodium), (2) exceeding the recommended initial dose of Lamictal, or (3) exceeding the recommended dose escalation for Lamictal. However, cases have occurred in the absence of these factors. Nearly all cases of life-threatening rashes caused by Lamictal have occurred within 2 to 8 weeks of treatment initiation. However, isolated cases have occurred after prolonged treatment (e.g., 6 months). Accordingly, duration of therapy cannot be relied upon as means to predict the potential risk heralded by the first appearance of a rash. Although benign rashes are also caused by Lamictal, it is not possible to predict reliably which rashes will prove to be serious or life threatening. Accordingly, Lamictal should ordinarily be discontinued at the first sign of rash, unless the rash is clearly not drug related. Discontinuation of treatment may not prevent a rash from becoming life threatening or permanently disabling or disfiguring Dizziness, headache, and ataxia were the most frequent adverse nervous system effects, occurring in 38, 29, and 22% of adults, respectively, in controlled trials of lamotrigine adjunctive therapy. The frequency of dizziness and ataxia, and the rate of discontinuance of lamotrigine because of these adverse effects, were dose related in clinical trials; in a dose-response study, dizziness occurred in 54, 31, or 27% of patients receiving lamotrigine 500 mg/day, lamotrigine 300 mg/day, or placebo, respectively, while ataxia occurred in 28, 10, or 10% of those receiving these respective regimens. Somnolence or insomnia occurred in 14 or 6%, respectively, of adults receiving lamotrigine as adjunctive therapy in controlled clinical trials. Incoordination or tremor was reported in 6 or 4%, respectively, of lamotrigine-treated adults ... Depression occurred in 4%, anxiety in 4%, irritability in 3%, speech disorder in 3%, and concentration disturbance in 2% of adults receiving lamotrigine as adjunctive therapy in controlled clinical trials. Seizure or seizure exacerbation has been reported in 3 or 2% of adults, respectively, receiving lamotrigine as adjunctive therapy in controlled trials; an increase in seizure frequency also has been reported with lamotrigine therapy. Treatment-emergent seizures diagnosed unequivocally as status epilepticus were reported in 7 of 2343 adults receiving adjunctive therapy with lamotrigine in clinical trials; however, the manufacturer states that valid estimates of the incidence of treatment-emergent status epilepticus are difficult to obtain because of variations in the definitions used by different investigators to identify such cases. Coordination abnormality, dizziness, anxiety, and insomnia occurred in 7, 7, 5, and 5% respectively, of adults receiving lamotrigine as monotherapy in a controlled trial; amnesia, ataxia, asthenia, depression, hypesthesia, libido increase, decreased or increased reflexes, nystagmus, and irritability each occurred in 2% of such patients. Paresthesia or asthenia occurred in more than 1% of adults receiving lamotrigine as adjunctive therapy in controlled clinical trials but with equal or greater frequency in those receiving placebo. For more Drug Warnings (Complete) data for LAMOTRIGINE (42 total), please visit the HSDB record page. Pharmacodynamics Lamotrigine likely prevents seizures and prevents mood symptoms via stabilizing presynaptic neuronal membranes and preventing the release of excitatory neurotransmitters such as glutamate, which contribute to seizure activity. A note on cardiovascular effects The metabolite of lamotrigine, 2-N-methyl metabolite (formed by glucuronidation), is reported to cause dose-dependent prolongations of the PR interval, widening of the QRS complex, and at higher doses, complete AV block. Although this harmful metabolite is only found in trace amounts in humans, plasma concentrations may increase in conditions that cause decreased drug glucuronidation, such as liver disease. |
| Molecular Formula |
C9H7CL2N5
|
|
|---|---|---|
| Molecular Weight |
256.09
|
|
| Exact Mass |
255.007
|
|
| Elemental Analysis |
C, 42.21; H, 2.76; Cl, 27.69; N, 27.35
|
|
| CAS # |
84057-84-1
|
|
| Related CAS # |
Lamotrigine-13C3,d3; 1246815-13-3; Lamotrigine-13C3; 1188265-38-4; Lamotrigine hydrate; 375347-20-9; Lamotrigine-13C,d3; 2517756-06-6; Lamotrigine-13C2,15N; 2483830-10-8; Lamotrigine-d3; 1132746-94-1
|
|
| PubChem CID |
3878
|
|
| Appearance |
White to off-white solid powder
|
|
| Density |
1.6±0.1 g/cm3
|
|
| Boiling Point |
503.1±60.0 °C at 760 mmHg
|
|
| Melting Point |
177-181°C
|
|
| Flash Point |
258.1±32.9 °C
|
|
| Vapour Pressure |
0.0±1.3 mmHg at 25°C
|
|
| Index of Refraction |
1.706
|
|
| LogP |
-0.19
|
|
| Hydrogen Bond Donor Count |
2
|
|
| Hydrogen Bond Acceptor Count |
5
|
|
| Rotatable Bond Count |
1
|
|
| Heavy Atom Count |
16
|
|
| Complexity |
242
|
|
| Defined Atom Stereocenter Count |
0
|
|
| SMILES |
ClC1C(Cl)=C(C2C(N)=NC(N)=NN=2)C=CC=1
|
|
| InChi Key |
PYZRQGJRPPTADH-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C9H7Cl2N5/c10-5-3-1-2-4(6(5)11)7-8(12)14-9(13)16-15-7/h1-3H,(H4,12,13,14,16)
|
|
| Chemical Name |
6-(2,3-dichlorophenyl)-1,2,4-triazine-3,5-diamine
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: This product requires protection from light (avoid light exposure) during transportation and storage. |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (9.76 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (9.76 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 2.5 mg/mL (9.76 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 4: 0.5% methylcellulose: 30 mg/mL |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.9049 mL | 19.5244 mL | 39.0488 mL | |
| 5 mM | 0.7810 mL | 3.9049 mL | 7.8098 mL | |
| 10 mM | 0.3905 mL | 1.9524 mL | 3.9049 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
Lithium Versus Lamotrigine in Bipolar Disorder, Type II
CTID: NCT06184581
Phase: Phase 4 Status: Recruiting
Date: 2024-06-07
 |
|---|
 |
 |