
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
Purity: ≥98%
JNJ-7777120 (JNJ7777120; JNJ 7777120), an indole analog, is a potent and selective non-imidazole-based histamine H4R receptor antagonist that can reduce allergic and asthmatic symptoms. It suppresses the histamine H4R receptor with a Ki of 4 ±1 nM. JNJ-7777120 was found by Johnson & Johnson Pharmaceuticals using an HTS/high throughput screen.
| Targets |
Histamine H4 receptor ( Ki = 4.5 nM )
|
||
|---|---|---|---|
| ln Vitro |
In vitro activity: JNJ 7777120 exhibits a high degree of affinity for the H4 receptor and possesses selectivity over other histamine receptors by more than 1000 times. A strong and selective H4 receptor antagonist, JNJ 7777120 has little to no affinity for more than 50 other targets.
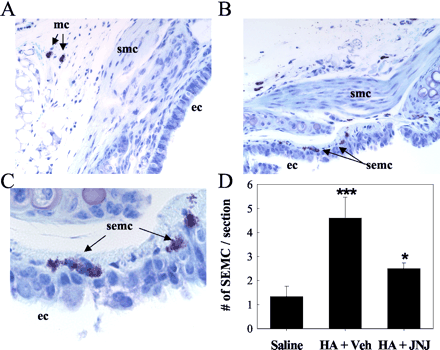
Effects of repeated administration of olopatadine and JNJ7777120 on histological changes and the number of mast cells [3] Histological examination revealed that the repeated treatment with PiCl caused severe acanthosis and the infiltration by inflammatory cells including lymphocytes and eosinophils of the ear lobe (Fig. 2B, E). The number of mast cells also increased significantly (Fig. 2G, I). Combined treatment with olopatadine and JNJ7777120 improved the hyperepidermis (Fig. 2C, F). JNJ7777120 decreased the number of mast cells (Fig. 2H, I). Olopatadine alone did not decrease but augmented the inhibitory effects of JNJ7777120 (Fig. 2I). Effects of olopatadine and JNJ7777120 on production of TARC and MDC in BMMC [3] To analyze the mechanism behind the anti-inflammatory effects of H1R and H4R antagonists, we tested whether olopatadine and/or JNJ7777120 inhibit TARC and MDC produced by BMMC. Bone marrow cells prepared from NC/Nga mice were induced to differentiate into FcεRI+/c-kit+ mast cells (more than 80%) over 9 days (Fig. 4A). The stimulation of BMMC with the antigen increased the levels of histamine (Fig. 4B), TARC, and MDC (Fig. 4C) in the medium collected at 24 h. The histamine release was slightly inhibited by olopatadine at 10 µM (about 16% inhibition) but not JNJ7777120 at 30 µM (data not shown). The production of TARC and MDC was inhibited slightly by olopatadine at 10 µM and significantly by JNJ7777120 in a dose-dependent manner (30–100 µM) (Fig. 4C). The H3R/H4R antagonist thioperamide (100 µM) also suppressed the production of TARC (Fig. 4D). Furthermore, JNJ7777120 plus olopatadine inhibited additively TARC production (Fig. 4D). It was unlikely that JNJ7777120 reduced the production of these cytokines via toxic effects because JNJ7777120 did not reduce the antigen-induced release of IL-13 (data not shown). Effects of olopatadine on histamine-induced down-regulation of Sema3A expression in PAM212 cells [3] Finally, we tested whether olopatadine and/or JNJ7777120 affect the level of mRNA for Sema3A, the regulatory factor of neuronal elongation. The expression of H1R was detected by real-time PCR, but H4R was not detected in unstimulated and histamine-stimulated PAM212 cells (data not shown). The level of mRNA for Sema3A was decreased by histamine at 10 µM (Fig. 5A). The level reached a minimum at 3 h and slowly recovered over the next 9 h (Fig. 5B). The histamine-induced reduction in the level of Sema3A mRNA was antagonized by olopatadine (Fig. 5C) but not by JNJ7777120 at 30 µM (data not shown). |
||
| ln Vivo |
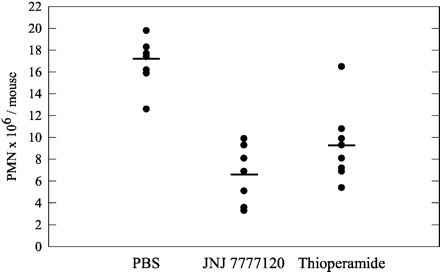
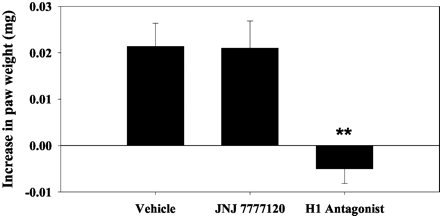
JNJ 7777120 exhibits a half-life of approximately 3 hours in both rats and dogs, with an oral bioavailability of approximately 30% in rats and 100% in dogs. JNJ 7777120 prevents calcium influx and histamine-induced chemotaxis in mast cells derived from mouse bone marrow. JNJ 7777120 has the ability to prevent histamine-induced tracheal mast cell migration in mice, which occurs from the connective tissue toward the epithelium. In a mouse model of peritonitis induced by zymosan, JNJ 7777120 significantly inhibits neutrophil infiltration.
JNJ7777120 can block the histamine-induced migration of tracheal mast cells from the connective tissue toward the epithelium in mice. JNJ 7777120 significantly blocks neutrophil infiltration in a mouse zymosan-induced peritonitis model. This model is reported to be mast cell-dependent, which suggests that the compound effect may be mediated by mast cells. These results indicate that the histamine H4 receptor plays a role in the inflammatory process. Selective H4 receptor antagonists like JNJ 7777120 may have the potential to be useful in treating inflammation in humans.[2] JNJ7777120 attenuated scratching behavior after a single administration and improved dermatitis, as assessed with clinical scores, pathology, and cytokine levels in skin lesions when administered repeatedly. These effects were augmented by combined treatment with olopatadine, having a similar therapeutic efficacy to prednisolone. JNJ7777120 inhibited dose-dependently the production of thymus and activation-regulated chemokine/CCL17 and macrophage-derived chemokine/CCL22 from antigen-stimulated BMMC. [3] Effects of single administration of olopatadine and JNJ7777120 on scratching behavior [3] Three days after the fifth challenge, drugs were administered and scratching counts were determined. As shown in Fig. 1E, the number of times the mice scratched during 2 h was significantly increased. Administration of olopatadine or JNJ7777120 apparently reduced the counts, and combined treatment significantly decreased them. The anti-pruritus effect of the combined treatment was as potent as that of prednisolone. Amelioration of dermatitis by repeated administration of olopatadine and JNJ7777120 [3] Olopatadine and/or JNJ7777120 were administered every other day after the fifth treatment with PiCl, and the dermatitis score was evaluated until week 10. The dermatitis score progressively increased dependent on the number of challenges with PiCl. JNJ7777120, but not olopatadine, prevented this increase (Fig. 1F). Interestingly, combined treatment had a significant effect from 8 weeks (Fig. 1F), and the severity of the dermatitis was reduced about 50% compared with the control group at 10 weeks. Consistent with the anti-pruritic effects, dual inhibition of H1R and H4R was as effective as prednisolone. Effects of repeated administration of olopatadine and JNJ7777120 on levels of cytokines in tissue and IgE in plasma [3] Next, we assessed the levels of IL-4, IL-5, TSLP, TARC, and NGF in tissue, and IgE in plasma at 10 weeks after the sensitization. Olopatadine decreased the level of NGF but not IL-4, IL-5, TSLP, TARC, or IgE (Fig. 3). In contrast, JNJ7777120 not only significantly decreased the NGF levels but also markedly inhibited the increases in IL-4, IL-5, TSLP, and TARC (Fig. 3). Although neither olopatadine nor JNJ7777120 alone reduced the plasma IgE level, in combination they inhibited the increase in IgE level similar to prednisolone (Fig. 3F). The production of MDC in tissue was not significantly inhibited by olopatadine, JNJ7777120, and prednisolone (data not shown). |
||
| Enzyme Assay |
[3 H]Histamine Binding [1]
Cell pellets from histamine H4-expressing SK-N-MC cells were homogenized in 20 mM TrisHCl/0.5 mM EDTA. Supernatants from an 800g spin were collected and recentrifuged at 30,000g for 30 min. Pellets were rehomogenized in 50 mM Tris/5 mM EDTA. Membranes were incubated with 5 nM [3 H]histamine with or without tested compounds for 45 min at 25 °C. Membranes were then filtered through GF/C membrane plates which were pretreated with 0.3% PEI and counted on a Topcount. Nonspecific binding was defined with 10 µM cold histamine. For competition binding studies using [3 H]histamine Ki values were calculated based on an experimentally determined Kd value of 5 nM and a ligand concentration of 5 nM according to Cheng and Prusoff. Cyclic AMP Accumulation [1] A subline of SK-N-MC cells was created that expressed both histamine H4 and a reporter gene construct. The reporter gene was β-galactosidase under the control of cyclic AMP responsive elements. Cells were plated in 96-well plates the night before the assay. To start the assay, agonists were added directly to the cell medium followed 10 min later by an addition of forskolin (10 µM final concentration). Cells were returned to the incubator for 6 h at 37 °C. The medium was then aspirated, and the cells were washed with 200 µL of PBS followed by a second aspiration. Cells were lysed with 25 µL of 0.1x assay buffer (10 mM sodium phosphate, pH 8, 0.2 mM MgSO4, 0.01 mM MnCl2) and incubated at rt for 10 min. Cells were then incubated for 10 min with 100 µL of 1x assay buffer containing 0.5% Triton and 40 mM betamercaptoethanol. Color was developed using 25 µM of 1 mg/ml substrate solution (chlorophenol red β-Dgalactopyranoside). Color was quantitated on a microplate reader at absorbance 570 nm. |
||
| Cell Assay |
Stimulation of BMMC with anti-DNP IgE and DNP-BSA, and measurement of levels of thymus and activation-regulated chemokine (TARC/CCL17) and macrophage-derived chemokine (MDC/CCL22)[3]
Bone marrow-derived mast cells were primed overnight with anti-DNP IgE (0.5 µg/ml). The cells were treated with olopatadine and/or JNJ7777120 for 30 min and then stimulated with DNP-BSA (1–25 ng/ml) for 24 h. The amount of histamine in the culture medium was determined by EIA, and TARC and MDC levels were measured with ELISA kits. |
||
| Animal Protocol |
|
||
| References | |||
| Additional Infomation |
Following the discovery of the human histamine H4 receptor, a high throughput screen of our corporate compound collection identified compound 6 as a potential lead. Investigation of the SAR resulted in the discovery of novel compounds 10e/JNJ7777120 and 10l, which are the first potent and selective histamine H4 receptor antagonists to be described.
A detailed biological evaluation of 10e and 10l was undertaken due to their high affinity for the H4 receptor. Functional activity versus the human H4 receptor was determined using SK-N-MC cells stably transfected with the human H4 receptor.8 In these cells, addition of histamine induces a decrease in the forskolin stimulated cAMP levels. Compounds 10e and 10l produced a rightward shift in the histamine dose response curve yielding a pA2 = 8.14 and 8.11, respectively, confirming that they function as H4 receptor antagonists. These compounds also showed high affinity for the rat histamine H4 receptor (JNJ7777120/10e Ki = 2.4 nM and 10lKi = 3.3 nM) and were found to be >1000-fold selective for the H4 receptor over the other histamine receptors. When tested against a panel of over 50 receptor targets representing the major classes of biogenic amine receptors, neuropeptide receptors, ion channel binding sites, and transporters, these compounds showed minimal biological activity. Conclusion. After screening our corporate compound collection against the histamine H4 receptor and identifying lead compound indolylpiperazine (6), we began a medicinal chemistry program to improve the biological activity of lead compound 6 and elucidate the SAR for the series. Several general trends can be gleaned from the results presented in Tables 1 and 2. Our SAR investigation suggested that in order to maintain potency less than 100 nM, substitution on the piperazine nitrogen must be limited to a methyl group. In contrast, a variety of substituents about the indole ring were well tolerated. In general, lipophilic groups or compact polar groups increased affinity for the H4 receptor relative to the unsubstituted analogue. Disubstitution on the indole ring was also tolerated, resulting in compounds with activity comparable to the 5-substituted analogues. Detailed biological evaluation of selected analogues, 10e and 10l, demonstrated that these ligands are selective for the histamine H4 receptor and that they function as receptor antagonists. Thus, we have prepared the first potent and selective non-imidazole histamine H4 antagonists. Further pharmacological characterization of 10e, JNJ7777120, will be reported in due course.[1] Background: Although histamine H1 receptor (H1R) antagonists are commonly used to treat atopic dermatitis, the treatment is not always effective. The histamine H4 receptor (H4R) was recently described as important to the pruritus in dermatitis. Here, we investigated whether the combination of a H1R antagonist plus a H4R antagonist attenuates chronic dermatitis in NC/Nga mice. Methods: Chronic dermatitis was developed by repeated challenges with picryl chloride on the dorsal back and ear lobes. The therapeutic effects of the H1R antagonist olopatadine and H4R antagonist JNJ7777120 on scratching and the severity of dermatitis were evaluated. In addition, the mechanisms responsible for the anti-allergic effects of H1R and/or H4R antagonism were examined using bone marrow-derived mast cells (BMMC) and keratinocytes. Results: JNJ7777120 attenuated scratching behavior after a single administration and improved dermatitis, as assessed with clinical scores, pathology, and cytokine levels in skin lesions when administered repeatedly. These effects were augmented by combined treatment with olopatadine, having a similar therapeutic efficacy to prednisolone. JNJ7777120 inhibited dose-dependently the production of thymus and activation-regulated chemokine/CCL17 and macrophage-derived chemokine/CCL22 from antigen-stimulated BMMC. In addition, olopatadine reversed the histamine-induced reduction of semaphorin 3A mRNA in keratinocytes. Conclusion: Combined treatment with H1R and H4R antagonists may have a significant therapeutic effect on chronic dermatitis through the synergistic inhibition of pruritus and skin inflammation.[3] |
| Molecular Formula |
C14H16CLN3O
|
|
|---|---|---|
| Molecular Weight |
277.75
|
|
| Exact Mass |
277.098
|
|
| Elemental Analysis |
C, 60.54; H, 5.81; Cl, 12.76; N, 15.13; O, 5.76
|
|
| CAS # |
459168-41-3
|
|
| Related CAS # |
|
|
| PubChem CID |
4908365
|
|
| Appearance |
White to off-white solid powder
|
|
| Density |
1.3±0.1 g/cm3
|
|
| Boiling Point |
477.0±45.0 °C at 760 mmHg
|
|
| Flash Point |
242.3±28.7 °C
|
|
| Vapour Pressure |
0.0±1.2 mmHg at 25°C
|
|
| Index of Refraction |
1.656
|
|
| LogP |
0.69
|
|
| Hydrogen Bond Donor Count |
1
|
|
| Hydrogen Bond Acceptor Count |
2
|
|
| Rotatable Bond Count |
1
|
|
| Heavy Atom Count |
19
|
|
| Complexity |
344
|
|
| Defined Atom Stereocenter Count |
0
|
|
| SMILES |
ClC1C([H])=C([H])C2=C(C=1[H])C([H])=C(C(N1C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])C1([H])[H])=O)N2[H]
|
|
| InChi Key |
HUQJRYMLJBBEDO-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C14H16ClN3O/c1-17-4-6-18(7-5-17)14(19)13-9-10-8-11(15)2-3-12(10)16-13/h2-3,8-9,16H,4-7H2,1H3
|
|
| Chemical Name |
(5-chloro-1H-indol-2-yl)-(4-methylpiperazin-1-yl)methanone
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (9.00 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (9.00 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 2.5 mg/mL (9.00 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 4: 30% Propylene glycol , 5% Tween 80 , 65% D5W: 20 mg/mL |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.6004 mL | 18.0018 mL | 36.0036 mL | |
| 5 mM | 0.7201 mL | 3.6004 mL | 7.2007 mL | |
| 10 mM | 0.3600 mL | 1.8002 mL | 3.6004 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
 |
|---|
  |
|