
| Size | Price | Stock | Qty |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
Purity: ≥98%
Ivabradine HCl (Corlentor; Corlanor; Coraxan; Ivabid; Procoralan; Coralan; S16257; S 16257; S-16257; Bradia), the hydrochloride salt of Ivabradine, is a novel and potent cardiac pacemaker current (If) inhibitor with antianginal effects. It suppresses IF with IC50 of 2.9 μM. Ivabradine is a pure heart rate reducer that works exclusively on the sinoatrial node's pacemaker activity. A novel medication called ivabradine is prescribed to treat stable angina pectoris symptomatically. In contrast to beta blockers and calcium channel blockers, two frequently prescribed antianginal medications, ivabradine works by selectively inhibiting the funny channel to lower heart rate.
| Targets |
Adrenergic Receptor
|
|
|---|---|---|
| ln Vitro |
|
|
| ln Vivo |
|
|
| Animal Protocol |
|
|
| References | ||
| Additional Infomation |
Ivabradine hydrochloride is a hydrochloride obtained by combining ivabradine with one molar equivalent of hydrochloric acid. Used to treat patients with angina who have intolerance to beta blockers and/or heart failure. It has a role as a cardiotonic drug. It contains an ivabradine(1+).
Ivabradine Hydrochloride is the hydrochloride salt form of ivabradine, an orally bioavailable, hyperpolarization-activated, cyclic nucleotide-gated (HCN) channel blocker, with negative chronotropic activity. Upon administration, ivabradine selectively binds to the intracellular portion of the HCN channel pore and blocks HCN channels in the pacemaker cells within the sinoatrial (SA) node. This inhibits the If (funny) pacemaker ion current, prevents the inward flow and intracellular accumulation of positively charged ions, reduces pacemaker activity and slows diastolic depolarization. This decreases heart rate, reduces myocardial oxygen demand and allows more time for blood to flow to the myocardium without affecting cardiac contractility. HCN channels, mixed sodium (Na+) and potassium (K+) channels that carry the inward If current, play a key role in the regulation of pacemaker firing rate in the SA node. The If pacemaker current, the inward flow of positively charged Na+-K+ ions, initiates the spontaneous diastolic depolarization phase and modulating heart rate. A benzazepine derivative and selective HYPERPOLARIZATION-ACTIVATED CYCLIC NUCLEOTIDE-GATED CHANNELS inhibitor that lowers the heart rate. It is used in the treatment of CHRONIC STABLE ANGINA in patients unable to take BETA-ADRENERGIC BLOCKERS, and in the treatment of HEART FAILURE. See also: Ivabradine (has active moiety). Drug Indication Symptomatic treatment of chronic stable angina pectoris Ivabradine is indicated for the symptomatic treatment of chronic stable angina pectoris in coronary artery disease adults with normal sinus rhythm and heart rate ⥠70 bpm. Ivabradine is indicated: in adults unable to tolerate or with a contra-indication to the use of beta-blockersorin combination with beta-blockers in patients inadequately controlled with an optimal beta-blocker dose. Treatment of chronic heart failure Ivabradine is indicated in chronic heart failure NYHA II to IV class with systolic dysfunction, in patients in sinus rhythm and whose heart rate is ⥠75 bpm, in combination with standard therapy including beta-blocker therapy or when beta-blocker therapy is contraindicated or not tolerated. Symptomatic treatment of chronic stable angina pectorisIvabradine is indicated for the symptomatic treatment of chronic stable angina pectoris in coronary artery disease adults with normal sinus rhythm and heart rate ⥠70 bpm. Ivabradine is indicated : - in adults unable to tolerate or with a contra-indication to the use of beta-blockers- or in combination with beta-blockers in patients inadequately controlled with an optimal beta-blocker dose. Treatment of chronic heart failureIvabradine is indicated in chronic heart failure NYHA II to IV class with systolic dysfunction, in patients in sinus rhythm and whose heart rate is ⥠75 bpm, in combination with standard therapy including beta-blocker therapy or when beta-blocker therapy is contraindicated or not tolerated. (see section 5. 1) Symptomatic treatment of chronic stable angina pectorisIvabradine is indicated for the symptomatic treatment of chronic stable angina pectoris in coronary artery disease adults with normal sinus rhythm and heart rate ⥠70 bpm. Ivabradine is indicated: in adults unable to tolerate or with a contraindication to the use of beta-blockersor in combination with beta-blockers in patients inadequately controlled with an optimal beta-blocker dose. Treatment of chronic heart failureIvabradine is indicated in chronic heart failure NYHA II to IV class with systolic dysfunction, in patients in sinus rhythm and whose heart rate is ⥠75 bpm, in combination with standard therapy including beta-blocker therapy or when beta-blocker therapy is contraindicated or not tolerated. Symptomatic treatment of chronic stable angina pectoris Ivabradine is indicated for the symptomatic treatment of chronic stable angina pectoris in coronary artery disease adults with normal sinus rhythm and heart rate ⥠70 bpm. Ivabradine is indicated : in adults unable to tolerate or with a contraindication to the use of beta-blockersor in combination with beta-blockers in patients inadequately controlled with an optimal beta-blocker dose. Treatment of chronic heart failure Ivabradine is indicated in chronic heart failure NYHA II to IV class with systolic dysfunction, in patients in sinus rhythm and whose heart rate is ⥠75 bpm, in combination with standard therapy including beta-blocker therapy or when beta-blocker therapy is contraindicated or not tolerated. Symptomatic treatment of chronic stable angina pectoris in coronary artery disease adults with normal sinus rhythm and heart rate ⥠70 bpm. Ivabradine is indicated: - in adults unable to tolerate or with a contra-indication to the use of beta-blockers - or in combination with beta-blockers in patients inadequately controlled with an optimal beta-blocker dose. Treatment of chronic heart failure Ivabradine is indicated in chronic heart failure NYHA II to IV class with systolic dysfunction, in patients in sinus rhythm and whose heart rate is ⥠75 bpm, in combination with standard therapy including beta-blocker therapy or when beta-blocker therapy is contraindicated or not tolerated. , Treatment of angina pectoris, Treatment of chronic heart failure, Treatment of coronary artery disease Treatment of angina pectoris, Treatment of chronic heart failure, Treatment of coronary artery disease |
| Molecular Formula |
C27H37CLN2O5
|
|
|---|---|---|
| Molecular Weight |
505.05
|
|
| Exact Mass |
504.239
|
|
| Elemental Analysis |
C, 64.21; H, 7.38; Cl, 7.02; N, 5.55; O, 15.84
|
|
| CAS # |
148849-67-6
|
|
| Related CAS # |
Ivabradine metabolite N-Demethyl Ivabradine hydrochloride; 1246638-08-3; Ivabradine-d6 hydrochloride; 2070009-63-9; Ivabradine; 155974-00-8; Ivabradine-d3 hydrochloride; 1217809-61-4; Ivabradine-d6; 1202000-62-1 (sulfate); 1086026-42-7 (oxalate); 1422274-66-5 (hemisulfate)
|
|
| PubChem CID |
3045381
|
|
| Appearance |
White to off-white solid powder
|
|
| Boiling Point |
626.9ºC at 760mmHg
|
|
| Melting Point |
193-196?C
|
|
| Flash Point |
332.9ºC
|
|
| Vapour Pressure |
1.24E-15mmHg at 25°C
|
|
| LogP |
4.049
|
|
| Hydrogen Bond Donor Count |
1
|
|
| Hydrogen Bond Acceptor Count |
6
|
|
| Rotatable Bond Count |
10
|
|
| Heavy Atom Count |
35
|
|
| Complexity |
663
|
|
| Defined Atom Stereocenter Count |
1
|
|
| SMILES |
Cl[H].O(C([H])([H])[H])C1=C(C([H])=C2C(=C1[H])[C@@]([H])(C([H])([H])N(C([H])([H])[H])C([H])([H])C([H])([H])C([H])([H])N1C(C([H])([H])C3=C([H])C(=C(C([H])=C3C([H])([H])C1([H])[H])OC([H])([H])[H])OC([H])([H])[H])=O)C2([H])[H])OC([H])([H])[H]
|
|
| InChi Key |
HLUKNZUABFFNQS-ZMBIFBSDSA-N
|
|
| InChi Code |
InChI=1S/C27H36N2O5.ClH/c1-28(17-21-11-20-14-25(33-4)26(34-5)16-22(20)21)8-6-9-29-10-7-18-12-23(31-2)24(32-3)13-19(18)15-27(29)30;/h12-14,16,21H,6-11,15,17H2,1-5H3;1H/t21-;/m1./s1
|
|
| Chemical Name |
3-[3-[[(7S)-3,4-dimethoxy-7-bicyclo[4.2.0]octa-1,3,5-trienyl]methyl-methylamino]propyl]-7,8-dimethoxy-2,5-dihydro-1H-3-benzazepin-4-one;hydrochloride
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (4.95 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (4.95 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: 50 mg/mL (99.00 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9800 mL | 9.9000 mL | 19.8000 mL | |
| 5 mM | 0.3960 mL | 1.9800 mL | 3.9600 mL | |
| 10 mM | 0.1980 mL | 0.9900 mL | 1.9800 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05973591 | Active Recruiting |
N/A | Dilated Cardiomyopathy Ventricular Remodeling |
Yonsei University | July 15, 2023 | N/A |
| NCT05882708 | Recruiting | Drug: Ivabradine | Sepsis Ivabradine Hemodynamics |
Second Affiliated Hospital of Guangzhou Medical University |
June 1, 2023 | Phase 4 |
| NCT03168529 | Recruiting | Drug: Placebo Drug: Ivabradine |
Heart Failure | Phillip Levy | July 1, 2018 | Phase 4 |
| NCT05348057 | Recruiting | Drug: Ivabradine | Cardiovascular Diseases | Qian geng | August 1, 2021 | Phase 4 |
| NCT05481177 | Recruiting | Drug: Ivabradine | Long Haul COVID Postural Orthostatic Tachycardia Syndrome |
Uniformed Services University of the Health Sciences |
June 14, 2023 | Phase 4 |
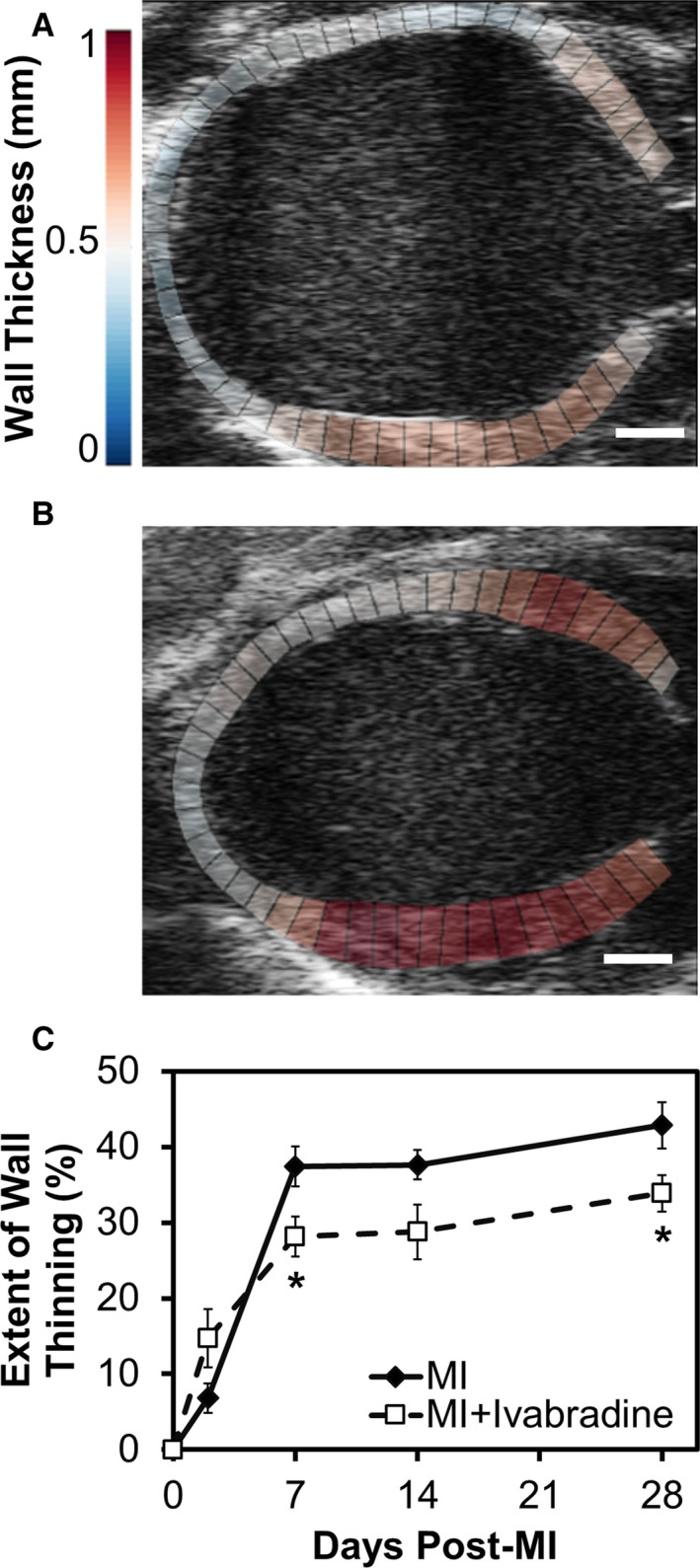
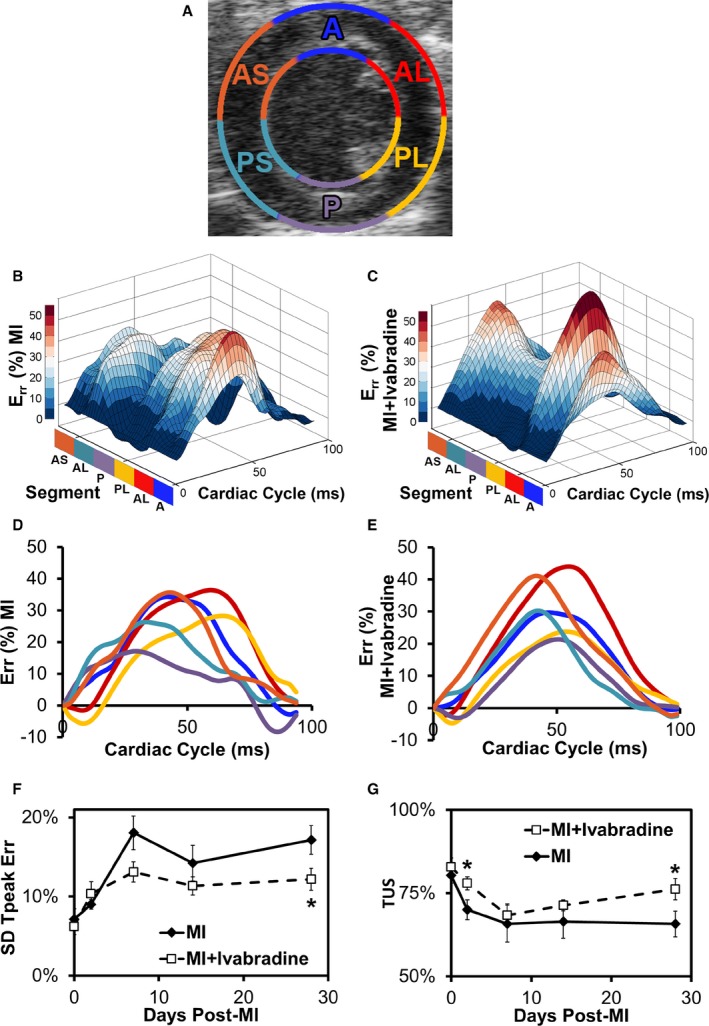
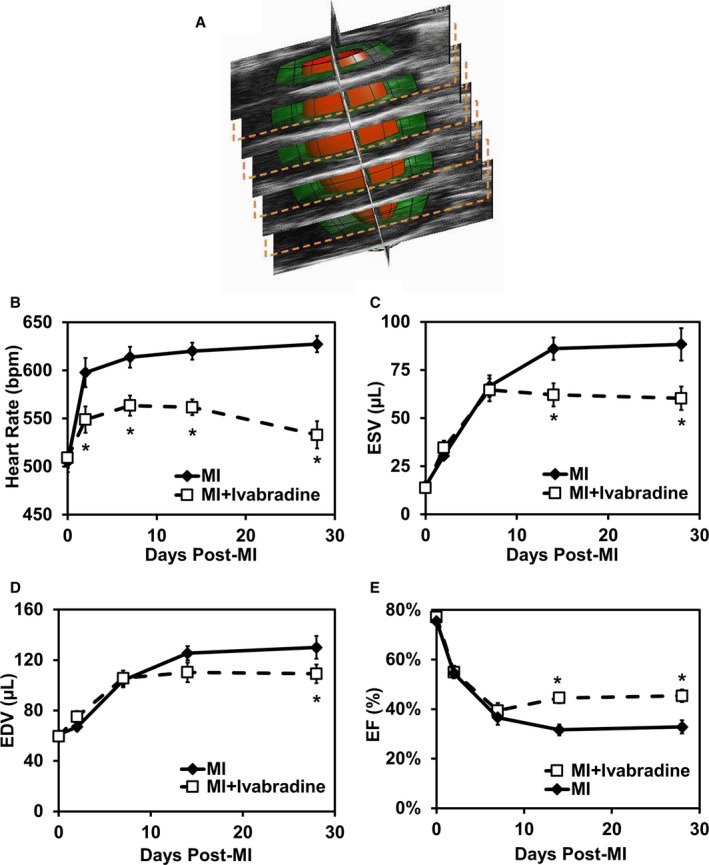 Ivabradine administration attenuates post‐MI LV remodeling.J Am Heart Assoc. 2016 Apr; 5(4): e002989. Ivabradine administration attenuates post‐MI LV remodeling.J Am Heart Assoc. 2016 Apr; 5(4): e002989. |
|---|
 |
 |