
| Size | Price | Stock | Qty |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g | |||
| 5g | |||
| Other Sizes |
Purity: ≥98%
Indacaterol Maleate (formerly known as QAB149; QAB-149; Onbrez Breezhale; arcapta neohaler; Onbrez), the maleate salt of indacaterol, is an approved anti-COPD (chronic obstructive pulmonary disease) drug acting as an ultra-long-acting β-adrenoceptor agonist developed by Novartis. β-adrenoceptor is activated with a pKi of 7.36 in. The FDA approved indacaterol in 2009 to treat chronic obstructive pulmonary disease (COPD). Using a dry powder inhaler, the aerosol formulation is administered. When Chinese hamster ovary cells are transfected with human β2 adrenoceptors and have a pEC50 of 8.06, indacaterol inhibits the production of cAMP.
| Targets |
β1-adrenoceptor ( pKi = 7.36 ); β2-adrenoceptor ( pKi = 5.48 )
|
||
|---|---|---|---|
| ln Vitro |
|
||
| ln Vivo |
|
||
| Cell Assay |
Cell Line: HT1080 cells (constitutively express MMP-9)
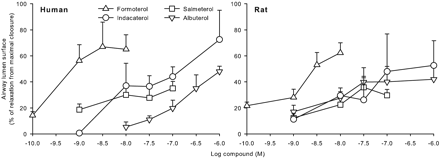
Concentration: 1, 2.5, 5, 10 µM Incubation Time: 12 h (pretreat) Result: Significantly suppressed MMP-9 mRNA expression in the 2.5 to 10 µM range. |
||
| Animal Protocol |
|
||
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
The median time to reach peak serum concentrations of indacaterol was approximately 15 minutes after single or repeated inhaled doses. Absolute bioavailability of indacaterol after an inhaled dose was on average 43-45%. Renal clearance plays a minor role (about 2 to 6% of systemic clearance) in the elimination of systemically available indacaterol. In a human ADME study where indacaterol was given orally, the fecal route of excretion was dominant over the urinary route. Indacaterol was excreted into human feces primarily as unchanged parent drug (54% of the dose) and, to a lesser extent, hydroxylated indacaterol metabolites (23% of the dose). After intravenous infusion the volume of distribution (Vz) of indacaterol was 2,361 L to 2,557 L indicating an extensive distribution. Renal clearance of indacaterol is, on average, between 0.46 and 1.2 L/h. Serum clearance of indacaterol is 18.8 L/h to 23.3 L/h. Metabolism / Metabolites After oral administration of radiolabeled indacaterol, unchanged indacaterol was the main component in serum, accounting for about one third of total drug-related AUC over 24 hours. The monohydroxylated derivative, glucuronide conjugate, and the 8-O-glucuronide were the most prominent metabolites in serum. Other metabolites identified include a diastereomer of the hydroxylated derivative, a N-glucuronide of indacaterol, and C- and N-dealkylated products. In vitro investigations indicated that UGT1A1 was the only UGT isoform that metabolized indacaterol to the phenolic O-glucuronide. CYP3A4 is the predominant isoenzyme responsible for hydroxylation of indacaterol. Indacaterol has known human metabolites that include (2S,3S,4S,5R)-6-[(1R)-2-[(5,6-diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1-(8-hydroxy-2-oxo-1H-quinolin-5-yl)ethoxy]-3,4,5-trihydroxyoxane-2-carboxylic acid. Biological Half-Life Indacaterol serum concentrations declined in a multi-phasic manner with an average terminal half-life ranging from 45.5 to 126 hours. The effective half-life, calculated from the accumulation of indacaterol after repeated dosing with once daily doses between 75 mcg and 600 mcg ranged from 40 to 56 hours which is consistent with the observed time-to-steady state of approximately 12-15 days. |
||
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Indacaterol is no longer marketed in the US. Although no published data exist on the use of indacaterol by mouth or inhaler during lactation, data from the related drug, terbutaline, indicate that very little is expected to be excreted into breastmilk. The authors of several reviews and expert guidelines agree that use of inhaled bronchodilators is acceptable during breastfeeding because of the low bioavailability and maternal serum levels after use. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding The in vitro human serum and plasma protein binding was 94.1-95.3% and 95.1-96.2%, respectively. |
||
| References | |||
| Additional Infomation |
Indacaterol is a monohydroxyquinoline that consists of 5-[(1R)-2-amino-1-hydroxyethyl]-8-hydroxyquinolin-2-one having a 5,6-diethylindan-2-yl group attached to the amino function. Used as the maleate salt for treatment of chronic obstructive pulmonary disease. It has a role as a beta-adrenergic agonist and a bronchodilator agent. It is a quinolone, a monohydroxyquinoline, a member of indanes, a secondary alcohol and a secondary amino compound. It is a conjugate base of an indacaterol(1+).
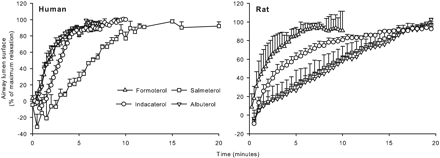
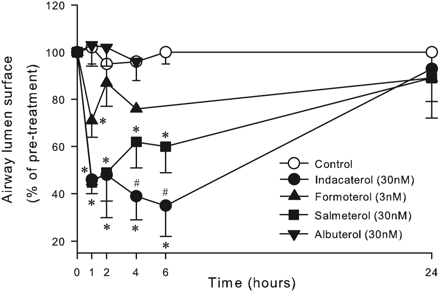
Indacaterol is a novel, ultra-long-acting, rapid onset β(2)-adrenoceptor agonist developed for Novartis for the once-daily management of asthma and chronic obstructive pulmonary disease. It was approved by the European Medicines Agency (EMA) on 30 November 2009 and by the FDA on 1 July 2011. It is marketed in Europe as Onbrez and in America as Arcapta Neohaler. Indacaterol is provided as its maleate salt form. Indacaterol is also a chiral molecule but only the pure R-enantiomer is dispensed. Indacaterol is a beta2-Adrenergic Agonist. The mechanism of action of indacaterol is as an Adrenergic beta2-Agonist. Drug Indication For the long term, once-daily-dosing maintenance of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema. FDA Label Onbrez Breezhaler is indicated for maintenance bronchodilator treatment of airflow obstruction in adult patients with chronic obstructive pulmonary disease. Oslif Breezhaler is indicated for maintenance bronchodilator treatment of airflow obstruction in adult patients with chronic obstructive pulmonary disease. Hirobriz Breezhaler is indicated for maintenance bronchodilator treatment of airflow obstruction in adult patients with chronic obstructive pulmonary disease. Mechanism of Action Indacaterol works by stimulating adrenergic beta-2 receptors in the smooth muscle of the airways. This causes relaxation of the muscle, thereby increasing the diameter of the airways, which become constricted in asthma and COPD. It is also long acting due to its high affinity to the lipid raft domains in the airway membrane so it slowly dissociates from the receptors. Indacaterol also has a high intrinsic efficacy so it is also very rapid acting - onset of action occurs within 5 minutes. The pharmacological effects of beta2-adrenoceptor agonist drugs, including indacaterol, are at least in part attributable to stimulation of intracellular adenyl cyclase, the enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3’, 5’-adenosine monophosphate (cyclic monophosphate). Increased cyclic AMP levels cause relaxation of bronchial smooth muscle. In vitro studies have shown that indacaterol has more than 24-fold greater agonist activity at beta2-receptors compared to beta1-receptors and 20-fold greater agonist activity compared to beta3-receptors. This selectivity profile is similar to formoterol. The clinical significance of these findings is unknown. |
| Molecular Formula |
C28H32N2O7
|
|---|---|
| Molecular Weight |
508.56
|
| Exact Mass |
508.22
|
| Elemental Analysis |
C, 66.13; H, 6.34; N, 5.51; O, 22.02
|
| CAS # |
753498-25-8
|
| Related CAS # |
Indacaterol; 312753-06-3
|
| PubChem CID |
6918554
|
| Appearance |
White to off-white solid powder
|
| Melting Point |
195-202°C with decomposition
195 °C (decomposition) |
| LogP |
3.663
|
| Hydrogen Bond Donor Count |
4
|
| Hydrogen Bond Acceptor Count |
4
|
| Rotatable Bond Count |
6
|
| Heavy Atom Count |
29
|
| Complexity |
589
|
| Defined Atom Stereocenter Count |
1
|
| SMILES |
O([H])[C@]([H])(C1C([H])=C([H])C(=C2C=1C([H])=C([H])C(N2[H])=O)O[H])C([H])([H])N([H])C1([H])C([H])([H])C2=C([H])C(C([H])([H])C([H])([H])[H])=C(C([H])([H])C([H])([H])[H])C([H])=C2C1([H])[H]
|
| InChi Key |
IREJFXIHXRZFER-PCBAQXHCSA-N
|
| InChi Code |
InChI=1S/C24H28N2O3.C4H4O4/c1-3-14-9-16-11-18(12-17(16)10-15(14)4-2)25-13-22(28)19-5-7-21(27)24-20(19)6-8-23(29)26-24;5-3(6)1-2-4(7)8/h5-10,18,22,25,27-28H,3-4,11-13H2,1-2H3,(H,26,29);1-2H,(H,5,6)(H,7,8)/b;2-1-/t22-;/m0./s1
|
| Chemical Name |
(Z)-but-2-enedioic acid;5-[(1R)-2-[(5,6-diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1-hydroxyethyl]-8-hydroxy-1H-quinolin-2-one
|
| Synonyms |
QAB149; Indacaterol maleate; QAB-149; QAB 149; trade name: Onbrez Breezhale; arcapta neohaler; Onbrez
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (4.09 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. Solubility in Formulation 2: ≥ 1.43 mg/mL (2.81 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 14.3 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 1.43 mg/mL (2.81 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 4: 4% DMSO+30% PEG 300+ddH2O: 10mg/mL |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9663 mL | 9.8317 mL | 19.6634 mL | |
| 5 mM | 0.3933 mL | 1.9663 mL | 3.9327 mL | |
| 10 mM | 0.1966 mL | 0.9832 mL | 1.9663 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT06035393 | Not yet recruiting | Drug: HRG2005 inhalation Drug: Placebo to match HRG2005 inhalation |
Chronic Obstructive Pulmonary Disease |
Jiangsu HengRui Medicine Co., Ltd. |
October 27, 2023 | Phase 2 |
| NCT01377051 | Completed | Drug: Indacaterol maleate Drug: Placebo |
COPD Dyspnea Hypoxemia |
University of Milan | May 2011 | Phase 4 |
| NCT01985334 | Completed | Drug: Glycopyrronium Drug: SABA Drug: LAMA |
COPD | Novartis Pharmaceuticals | February 14, 2014 | Phase 4 |
| NCT00556673 | Completed | Drug: indacaterol maleate/ mometasone furoate Drug: placebo to indacaterol maleate/ mometasone furoate |
Asthma | Novartis | October 2007 | Phase 2 |
| NCT00636961 | Completed | Drug: Indacaterol 300μg Drug: Placebo |
Chronic Obstructive Pulmonary Disease |
Novartis | February 2008 | Phase 2 |
 |
|---|
 |
 |