
| Size | Price | Stock | Qty |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g | |||
| Other Sizes |
Purity: ≥98%
Imatinib Mesylate (also known as STI571 mesylate; trade names: Gleevec or Glivec), the mesylate salt of imatinib, is an orally bioavailable, potent, multi-kinase inhibitor of v-Abl, c-Kit and PDGFR with potential antitumor activity. It inhibits the aforementioned kinases with IC50 values of 0.6 μM, 0.1 μM and 0.1 μM in cell-free and/or cell-based assays, respectively. Imatinib acts by binding to the intracellular domain located within tyrosine kinases (TK), thereby inhibiting ATP binding and preventing phosphorylation and the subsequent activation of growth receptors and their downstream signal transduction pathways. Imatinib inhibits tyrosine kinases encoded by the bcr-abl oncogene as well as receptor TKs encoded by the c-kit and platelet-derived growth factor receptor (PDGFR) oncogenes.
| Targets |
c-Kit (IC50 ~100 nM); Bcr-Abl (IC50 ~100 nM); PDGFR (IC50 ~100 nM)
|
|---|---|
| ln Vitro |
Imatinib (STI571) Mesylate prevents c-Kit autophosphorylation, MAPK activation, and Akt activation without changing the overall amounts of c-kit, MAPK, or Akt protein. About 100 nM is the concentration that results in 50% inhibition for these effects[1]. The kinase Bcr-Abl that causes chronic myeloid leukemia is highly susceptible to imatinib (STI571) mesylate (in vitro IC50 of 25 nM). Moreover, imatinib effectively inhibits PDGFR (in vitro IC50, 380 nM) and Kit (in vitro IC50, 410 nM)[2]. Imatinib (STI571) mesylate is a multi-target inhibitor of v-Abl, c-Kit, and it also inhibits the native PDGFβ receptor, Bcr/Abl, v-Abl, Tel/Abl, and c-Kit. However, it does not inhibit the EGFR, c-Fms, Flt3, Src family kinases, or numerous other tyrosine kinases. Imatinib has no effect on untransformed Ba/F3 cells growing in IL-3 or on Ba/F3 cells transformed by Tel/JAK2[3]. However, it inhibits the tyrosine phosphorylation and cell growth of Ba/F3 cells expressing Bcr/Abl, Tel/Abl, Tel/PDGFβR, and Tel/Arg with an IC50 of approximately 0.5 μM in each case. Imatinib mesylate specifically impedes c-Kit, PDGFR kinase, and Bcr/Abl activity. In patients with Philadelphia-positive (Ph+) acute lymphoblastic leukemia (ALL) and chronic myelogenous leukemia (CML), imatinib mesylate exhibits unique and swift antileukemic activity[4].
|
| ln Vivo |
Imatinib has varying antitumor effects on three xenografted tumors made from surgical samples of newly diagnosed human small cell lung cancers: the growth of the SCLC6, SCLC61, and SCLC108 tumors is inhibited by 80%, 40%, and 78%, respectively, while the growth of SCLC74 is not significantly affected. When administered by gavage at 10, 20, and 40 mg/kg, respectively, Imatinib significantly reduces the high fat-induced lipid staining area in ApoE(-/-) mice fed a high fat diet by 30%, 27%, and 35% compared to high fat diet untreated controls and suppresses carotid artery lipid accumulation.
|
| Enzyme Assay |
Rabbit antiserum is used to immunoprecipitate the PDGF receptor from extracts of BALB/c 3T3 cells, which is then left on ice for two hours. Antigen-antibody complexes are gathered using protein A-Sepharose beads. TNET (50 mM Tris, pH 7.5, 140 mM NaCl, 5 mM EDTA, 1% Triton X-100), TNE (50 mM Tris, pH 7.5, 140 mM EDTA), and kinase buffer (20 mM Tris, pH 7.5, 10 mM MgCl2) are the three solutions used to wash the immunoprecipitates twice. A variety of drug concentrations are added to the reaction mixture after PDGF (50 ng/mL) stimulation for 10 minutes at 4°C.
|
| Cell Assay |
Twenty-four hours before the test compounds are added, tested A549 cells are arranged at a density of 5×103 cells per well in 96-well flat-bottom plates. In addition to different doses of Imatinib mesylate (10, 100, 1000, and 10,000 ng/mL) and other cytostatic medications (Docetaxel (DTX) or Idarubicin (ID): 0.1, 1, 10, 100 ng/mL; Cisplatin (CIS): 1, 10, 100, 1000 ng/mL), the cells are incubated with PRI-2191 at two different concentrations (10 and 100 nM) for 96 hours. The assay known as sulforhodamine B (SRB) is utilized to assess the cytotoxic effect. As a result, the Dmitry Nevozhay software Cheburator 0.4 calculates the IC50 for every individual experiment[4].
|
| Animal Protocol |
Mice: We use female NOD/SCID mice that are 12–16 weeks old and weigh 20–25 g. On Day 0, mice receive a subcutaneous (s.c.) inoculation of A549 tumor cells suspension (5×106 cells in 0.2 mL of Hank's medium per mouse). Following this, they are randomly assigned to groups that receive different combinations of vitamin D analogs and chemotherapeutics. In the corresponding experiments, one of the two experimental protocols is used: 1. After the tumor cells are injected, treatment begins on Day 7 (when the tumors become palpable). For 19 days (from Days 7 to 25), imatinib mesylate is given intraperitoneally (i.p.) at a dose of 75 mg/kg/day. PRI-2191 is given orally or s.c. three times a week (on Days 7, 12, 14, 16, 19, 21, and 23) at a dose of 2 μg/kg/day. 2. After tumor cells are injected, treatment begins on Day 7 (when tumors become palpable). For 13 days (from Days 7-19), imatinib mesylate is given intraperitoneally (i.p.) at a dose of 50 mg/kg/day. PRI-2191 and PRI-2205 are given subcutaneously (s.c.) three times a week (on Days 7, 10, 12, 14, 17, 19, 21, 24, and 26) at doses of 1 or 10 μg/kg/day, respectively. Blood is drawn while the mice are sedated at the conclusion of the trials, and they are then killed.
Rats: In the experiments, male Lewis rats weighing between 270 and 320 g are employed. The Imatinib group (n = 7) receives an intraperitoneal injection of Imatinib mesylate (50 mg/kg), while the vehicle group (n = 7) receives 0.5 mL of 20% DMSO without Imatinib. Preliminary testing reveals that the 25 mg/kg dose slightly improves lung function without reaching statistical significance. Based on previous reports and this result, the intraperitoneal administration of 50 mg/kg was chosen. The animals have a left thoracotomy, and a tiny metallic clamp is used to occlude the left hilum. The occlusion is carried out 20 minutes following the administration of imatinib or the vehicle. Tidal volume (TV) and respiratory rate (RR) are set to 8 mL/kg and 80 breaths/min, respectively, during clamping. The clamp is taken off after 90 minutes of ischemia, and reperfusion is sustained for an additional 120 minutes. The bilateral lung's blood flow and ventilation are restored during reperfusion. The animals in the sham group (n=6) undergo 210 minutes of ventilation, thoracotomy, and heparinization. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
Imatinib is well absorbed after oral administration with Cmax achieved within 2-4 hours post-dose. Mean absolute bioavailability for the capsule formulation is 98%. Following oral administration in healthy volunteers, the elimination half-lives of imitanib and its major active metabolite, the N-desmethyl derivative, were approximately 18 and 40 hours, respectively. Mean imatinib AUC increased proportionally with increasing dose in the range 25 mg-1000 mg. There was no signficant change in the pharmacokinetics of imatinib on repeated dosing, and accumulation is 1.5-2.5 fold at steady state when Gleevec is dosed once daily. At clinically relevant concentrations of imatinib, binding to plasma proteins in in vitro experiments is approximately 95%, mostly to albumin and (alpha)1-acid glycoprotein. Fecal /elimination/ - 68% within 7 days (20% of dose unchanged); Renal /elimination/ - 13% within 7 days (5% of dose unchanged). Typically, clearance of imitanib in a 50-year-old patient weighing 50 kg is expected to be 8 L/hr, while for a 50-year-old patient weighing 100 kg the clearance will increase to 14 L/hr. However, the inter-patient variability of 40% in clearance does not warrant initial dose adjustment based on body weight and/or age but indicates the need for close monitoring for treatment related toxicity. In lactating female rats administered 100 mg/kg ... imatinib and/or its metabolites were extensively excreted in milk. It is estimated that approximately 1.% of a maternal dose is excreted into milk, which is equivalent to a dose to the infant of 30% the maternal dose per unit body weight. Metabolism / Metabolites CYP3A4 is the major enzyme responsible for metabolism of imatinib. Other cytochrome P450 enzymes, such as CYP1A2, CYP2D6, CYP2C9, and CYP2C19, play a minor role in its metabolism. The main circulating active metabolite in humans is the N-demethylated piperazine derivative, formed predominantly by CYP3A4. It shows in vitro potency similar to imatinib. The plasma AUC for this metabolite is about 15% of the AUC for imatinib. Biological Half-Life Elimination - Approximately 18 and 40 hours, for imatinib and its primary metabolite, respectively. |
| Toxicity/Toxicokinetics |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Limited information indicates that maternal doses of imatinib up to 400 mg daily produce low levels of the drug and its active metabolite in milk. Although a few breastfed infants apparently experienced no adverse effects during maternal use of imatinib, no long-term data are available. Until more data are available, imatinib should be used only with careful monitoring during breastfeeding. National Comprehensive Cancer Network guidelines, the manufacturer and some authors recommend that breastfeeding be discontinued during imatinib therapy and for 1 month after therapy. ◉ Effects in Breastfed Infants A woman receiving oral imatinib 400 mg daily for chronic myeloid leukemia breastfed her infant. No adverse effects were noted in the infant during the first 2 months of nursing. One woman with chronic myelogenous leukemia received imatinib 400 mg daily throughout pregnancy and during breastfeeding (extent not stated) for nearly 6 months postpartum. Her infant reportedly grew and developed normally. A woman with chronic myeloid leukemia received imatinib 400 mg daily starting at week 8 of pregnancy and continuing throughout 8 months of breastfeeding (extent not stated). The infant was healthy, but an atrial septal defect was repaired at 30 months of age. It was thought to be unrelated to imatinib therapy. A pregnant woman with Philadelphia chromosome-positive chronic myelogenous leukemia was started on imatinib 400 mg daily during pregnancy. After delivery, her preterm infant was fed colostrum until the middle of the fifth day postpartum when exclusive formula feeding was instituted. The infant was treated for apnea of prematurity and discharged on day 25 of life. No adverse effects on growth or development were noted during the first year of life. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Interactions In this study /an investigation was conducted to study/ the effect of concomitant administration of imatinib and idarubicin, an anthracycline with haematosuppressive activity, in nu/nu mice and murine bone marrow cells. Double-treated animals showed significantly increased mortality compared to mice that received imatinib or idarubicin alone only when idarubicin and imatinib were given simultaneously. The combined treatment induced a more severe neutropenia with a slower recovery when compared to mice treated with idarubicin alone. The myeloid metaplasia usually observed in the spleen after idarubicin treatment was absent in mice co-treated with imatinib. Bone marrow from double-treated animals also showed decreased numbers of megakaryocytes and myeloid precursor cells. In vitro culture of murine bone marrow cells in the presence of imatinib inhibited SCF-induced proliferation and recovery from treatment with idarubicin. ... Results indicate that the simultaneous administration of imatinib enhances idarubicin-induced hematopoietic toxicity in vivo and in vitro. Caution is recommended when administering Gleevec /imatinib/ with inhibitors of the CYP3A4 family (e.g., ketoconazole, itraconazole, erythromycin, clarithromycin). Substances that inhibit the cytochrome P450 isoenzyme (CYP3A4) activity may decrease metabolism and increase imatinib concetrations. Substances that are inducers of CYP3A4 activity may increase metabolism and decrease imatinib plasma concentrations. Co-medications that induce CYP3A4 (e.g. dexamethasone, phenytoin, carbamazepine, rifampicin, phenobarbital, or St. John's Wort) may reduce exposure to Gleevec /imatinib/. ...A patient on chronic therapy with phenytoin... given 350 mg daily dose of Gleevec had an AUC0-24 about one-fifth of the typical AUC0-24 of 20 ug/hr/mL. This probably reflects the induction of CYP3A4 by phenytoin. Imatinib increases the mean Cmax and AUC of simvastatin (CYP3A4 substrate) 2- and 3.5-fold, respectively, suggesting an inhibition of the CYP3A4 by imatinib. Particular caution is recommended when administering Gleevec /imatinib/ with CYP3A4 substrates that have a narrow therapeutic window (e.g., cyclosporine or pimozide). Gleevec will increase plasma concentration of other CYP3A4 metabolized drugs (e.g., triazolo-benzodiazepines, dihydropyridine calcium channel blockers, certain HMG-CoA reductase inhibitors, etc.). For more Interactions (Complete) data for IMATINIB MESYLATE (6 total), please visit the HSDB record page. |
| References | |
| Additional Infomation |
Therapeutic Uses
Imatinib mesylate (Gleevec), ... /an/ inhibitor of abl, kit, and platelet-derived growth factor receptor (PDGFR) tyrosine kinases, has been reported to be effective in the treatment of hypereosinophilic syndrome (HES) and a rare eosinophilia-associated chronic myeloid disorder (eos-CMD) characterized by the t(5;12)(q33;p13) cytogenetic abnormality. In the current study, we sought to confirm the preliminary observations in HES as well as evaluate the therapeutic value of imatinib in eos-CMD that is not associated with t(5;12)(q33;p13). Five patients with HES (all men, median age = 46 years) and 2 with eos-CMD (both men, aged 45 and 58 years) were treated with imatinib at a starting dose of 100 to 400 mg/day. Cytogenetic studies showed no evidence of either the bcr-abl translocation or t(5;12)(q33;p13) in any patient. Screening of exons encoding the intracellular catalytic domains and extracellular ligand binding domains of PDGFR beta (exons 2-23) and c-kit (exons 1-21) in six patients demonstrated mostly previously known polymorphisms. At a median follow-up of 17 weeks (range, 10-33 weeks), 2 patients with HES and 1 with eos-CMD have achieved complete clinical remission and 1 additional patient with HES has achieved a partial remission. In contrast to previous observations, all four responding patients had elevated serum interleukin-5 levels. /A study was conducted to include/ 28 patients with accelerated phase chronic myelogenous leukemia (CML) ... . Diagnosis of accelerated phase CML was based on karyotypic evolution (n = 9) and hematologic criteria (n = 18). All patients were begun on 600 mg/day of imatinib mesylate. Dose reductions to 400 mg/day and then 300 mg/day were prescribed for an absolute neutrophil count (ANC) of <0.5/microl or a platelet count of <20,000/microl. Twenty-seven of the 28 patients continued treatment for a median of 34 weeks. Eleven patients developed thrombocytopenia following an average of 8.4 +/- 1.4 weeks of therapy. The onset of thrombocytopenia was associated with disease progression in one patient and a decline in bone marrow megakaryocytes in the other 10. Nine patients recovered to a platelet count of >20,000/microl after an average of 19.7 +/- 1.8 weeks. Patients who developed thrombocytopenia had a longer duration of disease (9.39 vs. 4.35 years; P < 0.01) and were more likely to be diagnosed with accelerated phase CML by hematologic criteria. Hematologic responses in patients with and without thrombocytopenia were comparable; however, 31.3% of patients without thrombocytopenia had a complete cytogenetic response compared to none of those with thrombocytopenia. Grade III-IV thrombocytopenia is common in accelerated phase CML and may be a marker for the inability to achieve cytogenetic response using single agent imatinib mesylate. Imatinib is indicated for the treatment of gastrointestinal stromal tumors (GISTs). /NOT included in US product labeling/ Imatinib is indicated for the treatment of patients with chronic myeloid leukemia (MCL) in blast crisis; accelerated phase, or in chronic phase after failure of interferon-alpha therapy. (NOTE: Effectiveness is based on overall hematologic and cytogenetic response rates. There are no controlled trials demonstrating a clinical benefit, such as improvement in disease-related symptoms or increased survival.) /Included in US product labeling/ Imatinib mesylate (STI571, Gleevec, Glivec, a selective inhibitor of the BCR-ABL tyrosine kinase causative of chronic myeloid leukemia (CML), represents the paradigm of how a better understanding of the pathogenetic mechanisms of a neoplastic disease can lead to the development of a targeted molecular therapy. Phase II clinical trials have shown marked therapeutic activity of imatinib in all evolutive phases of CML, but notably in the chronic phase, where it induces complete hematological responses in almost 100% of patients resistant or intolerant to interferon, with a major cytogenetic response rate of 60%, including 41% complete cytogenetic responses. The preliminary results of an ongoing phase III multicenter randomized study comparing imatinib with interferon plus cytarabine as first-line treatment for CML favor imatinib in terms of efficacy and safety. If confirmed with longer follow-up,these results would establish imatinib as the choice therapy for the majority of CML patients, with allogeneic transplantation being restricted as initial therapy only to younger patients with a family donor. Drug Warnings Imatinib mesylate blocks bcr/abl kinase activity effectively, and thus is a promising drug in Philadelphia chromosome positive leukemias. While under imatinib treatment high hematological and cytogenetic response rates could be observed, usually only mild non-hematological side-effects like skin rash, edema, and muscular cramps occur. ... Two severe cases of acute generalized exanthematous pustulosis due to imatinib /are reported/. In both patients the generalized pustular eruptions could be observed 12 wk after initiation of imatinib treatment. Numerous microbiological investigations excluded an infectious etiology, and histopathology of cutaneous lesions was consistent with acute generalized exanthematous pustulosis. ... Withdrawal of imatinib led to a restitution at integrum of the integument. ... A tyrosine kinase inhibitor (STI571, Gleevec) has recently been applied in the treatment of chronic myeloid leukemia. /A/ ... case of pityriasis rosea occurring as a reaction to Gleevec in a woman with blast crisis of this disorder /is detailed/. Imatinib or STI 571 is ... a member of a new class of drugs known as signal transduction inhibitors. These compounds specifically inhibit the proliferation of v-abl- and bcr-abl-expressing cells and have recently been approved as treatment for chronic myeloid leukaemia (CML). ... An erosive oral lichenoid eruption confined to the buccal mucosa and dorsum of the tongue which appeared 12 weeks after commencement of imatinib in a 72-year-old woman with CML /is presented/. The histology was consistent with a lichenoid drug eruption. The lesions resolved upon withdrawal of the drug. Adverse effects occurring in 10% or more of patients include nausea, vomiting, edema, muscle cramps, diarrhea, GI or CNS hemorrhage, musculoskeletal pain, rash, headache, fatigue, arthralgia, dyspepsia, myalgia, weight increase, pyrexia, abdominal pain, cough, dyspnea, anorexia, constipation, nasopharyngitis, night sweats, pruritus, epistaxis, hypokalemia, petechiae, pneumonia, and weakness. For more Drug Warnings (Complete) data for IMATINIB MESYLATE (11 total), please visit the HSDB record page. |
| Molecular Formula |
C30H35N7O4S
|
|---|---|
| Molecular Weight |
589.71
|
| Exact Mass |
589.247
|
| Elemental Analysis |
C, 61.10; H, 5.98; N, 16.63; O, 10.85; S, 5.44
|
| CAS # |
220127-57-1
|
| Related CAS # |
Imatinib;152459-95-5;N-Desmethyl imatinib;404844-02-6
|
| PubChem CID |
123596
|
| Appearance |
white to off-white to brownish or yellowish tinged crystalline powder
|
| Density |
0.858 g/mL at 25 °C(lit.)
|
| Boiling Point |
133-134 °C(lit.)
|
| Melting Point |
214-224°C
|
| Flash Point |
64°F
|
| Index of Refraction |
n20/D 1.401(lit.)
|
| LogP |
5.196
|
| Hydrogen Bond Donor Count |
3
|
| Hydrogen Bond Acceptor Count |
10
|
| Rotatable Bond Count |
7
|
| Heavy Atom Count |
42
|
| Complexity |
799
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
S(C([H])([H])[H])(=O)(=O)O[H].O=C(C1C([H])=C([H])C(=C([H])C=1[H])C([H])([H])N1C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])C1([H])[H])N([H])C1C([H])=C([H])C(C([H])([H])[H])=C(C=1[H])N([H])C1=NC([H])=C([H])C(C2=C([H])N=C([H])C([H])=C2[H])=N1
|
| InChi Key |
YLMAHDNUQAMNNX-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C29H31N7O.CH4O3S/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36;1-5(2,3)4/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34);1H3,(H,2,3,4)
|
| Chemical Name |
methanesulfonic acid;4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl]benzamide
|
| Synonyms |
STI571; CGP-57148B; ST-1571 Mesylate; CGP 57148; CGP57148; CGP-57148; CGP-57148B; CGP57148B; ; STI-571; STI 571; Imatinib mesylate; Brand name: Gleevec (USA); Glivec (other countries)
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.08 mg/mL (3.53 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (3.53 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 2.08 mg/mL (3.53 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 4: Saline: 30 mg/mL Solubility in Formulation 5: 100 mg/mL (169.57 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication. |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6957 mL | 8.4787 mL | 16.9575 mL | |
| 5 mM | 0.3391 mL | 1.6957 mL | 3.3915 mL | |
| 10 mM | 0.1696 mL | 0.8479 mL | 1.6957 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
Ruxolitinib in Treating Participants With Chronic Myeloid Leukemia With Minimal Residual Disease While on Therapy With Tyrosine Kinase Inhibitors
CTID: NCT01751425
Phase: Phase 1 Status: Terminated
Date: 2024-08-26
|
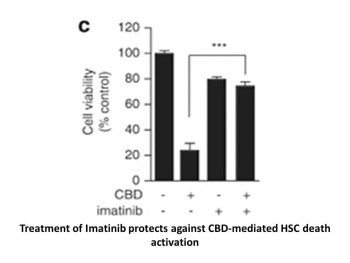 |
Effect of compounds 1 (Imatinib), 2 (Sunitinib), and 35 on cKIT mediated signaling pathways in GIST-T1 and GIST-5R cancer cell lines.J Med Chem.2016 Sep 22;59(18):8456-72. |