
| Size | Price | Stock | Qty |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
Purity: =99.62%
Gefitinib (formerly ZD1839, ZD-1839 or trade name: Iressa) is a potent and orally bioavailable EGFR inhibitor with potential anticancer activity. In the NR6wtEGFR and NR6W cells, it inhibits EGFR Tyr1173, Tyr992, Tyr1173, and Tyr992 with IC50 values of 37 nM, 37 nM, 26 nM, and 57 nM, respectively. A variety of human tumor types, such as head and neck, prostate, breast, ovarian, colon, small-cell lung, and non-small-cell lung cancer, show anti-angiogenic properties when treated with gefitinib. The FDA authorized gefitinib in May 2003 for the treatment of non-small cell lung cancer (NSCLC). As a third-line therapy, it was authorized for use as monotherapy in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) following the failure of both platinum-based and docetaxel chemotherapies.
| Targets |
Tyr1173 (IC50 = 26 nM); Tyr1173 (IC50 = 37 nM); Tyr992 (IC50 = 37 nM); Tyr992 (IC50 = 57 nM)
|
|---|---|
| ln Vitro |
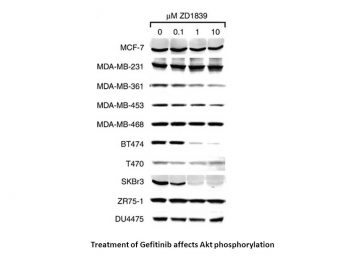
Gefitinib effectively inhibits all EGFR tyrosine phosphorylation sites in cell lines that express EGFR, including NR6, NR6M, and NR6W cell lines, as well as high and low-EGFR-expressing cell lines. Tyr992 and Tyr1173, two phosphorylation sites, are less sensitive and require greater concentrations of Gefitinib to inhibit. With an IC50 of 27 nM, gefitinib efficiently prevents PLC-γ phosphorylation in NR6W cells. PLC-γ phosphorylation is low in the NR6wtEGFR and NR6M cell lines, but it is more resistant to gefitinib inhibition in the latter, with IC50 values of 43 nM and 369 nM, respectively. Gefitinib inhibits Akt phosphorylations in the low-EGFR- and EGFRvIII-expressing cell lines, with IC50 values of 220 and 263 nM, respectively. When administered at doses ranging from 0.1 to 0.5μM, gefitinib significantly promotes NR6M cell colony formation as opposed to inhibiting it. On the other hand, Gefitinib totally prevents NR6M colony formation at a concentration of 2 μM. In both the high- and low-EGFR-expressing cell lines, gefitinib quickly and dose-dependently inhibits EGFR and ERK phosphorylation for up to 72 hours following EGF stimulation.[1] These EGF-driven untransformed MCF10A cells grow monolayerically when exposed to gefitinib, with an IC50 of 20 nM. [2] When paired with irradiation, Gefitinib (0.2 μM and 0.5 μM) significantly inhibited the growth of LoVo cells in comparison to radiation alone.[3]
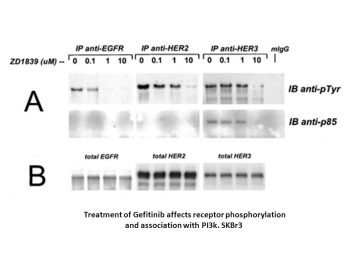
Epidermal growth factor receptor (EGFR) is frequently amplified and/or mutated in a number of human tumours and abnormal signalling from this receptor is believed to contribute to the malignant phenotype seen in these tumours. Gefitinib is a small molecule inhibitor that specifically binds and inhibits the EGFR tyrosine kinase and has been shown to inhibit the growth, proliferation, survival and invasion of a range of tumour cells overexpressing EGFR. However, clinical response to gefitinib has failed to correlate with EGFR levels and activity, indicating that other molecular mechanisms such as downstream signalling and mutations could be of importance in predicting clinical response. We therefore investigated the effect of the specific EGFR inhibitor gefitinib on the phosphorylation level, signalling and growth of cells expressing the naturally occurring constitutively active EGFR variant EGFRvIII, a low nontransforming level of EGFR and a high transforming level of EGFR. Results show that levels of gefitinib sufficient to suppress EGFR phosphorylations, EGFR-mediated proliferation and EGFR-mediated anchorage-independent growth are not sufficient to inhibit these features in cells expressing EGFRvIII. Furthermore, the data indicate that long-term exposure of EGFRvIII-expressing cells to low concentrations of gefitinib (0.01-0.1 microM) result in increased phosphotyrosine load of the receptor, increased signalling to ERK and stimulation of proliferation and anchorage-independent growth, presumably by inducing EGFRvIII dimerisation. Higher concentrations of gefitinib (1-2 microM), on the other hand, significantly decreased EGFRvIII phosphotyrosine load, EGFRvIII-mediated proliferation and anchorage-independent growth. Further studies are needed to investigate the implications of these important findings in the clinical setting.[1] The epidermal growth factor receptor (EGFR) is commonly overexpressed in many human tumors and provides a new target for anticancer drug development. ZD1839 ("Iressa"), a quinazoline tyrosine kinase inhibitor selective for the EGFR, has shown good activity in preclinical studies and in the early phase of clinical trials. However, because it remains unclear which tumor types are the best targets for treatment with this agent, the molecular characteristics that correlate with tumor sensitivity to ZD1839 have been studied. In a panel of human breast cancer and other epithelial tumor cell lines, HER2-overexpressing tumors were particularly sensitive to ZD1839. Growth inhibition of these tumor cell lines was associated with the dephosphorylation of EGFR, HER2, and HER3, accompanied by the loss of association of HER3 with phosphatidylinositol 3-kinase, and down-regulation of Akt activity. These studies suggest that HER2-overexpressing tumors are particularly susceptible to the inhibition of HER family tyrosine kinase signaling and suggest novel strategies to treat these particularly aggressive tumors.[2] Transforming growth factor alpha (TGF-alpha) is an autocrine growth factor for human cancer. Overexpression of TGF-alpha and its specific receptor, the epidermal growth factor receptor (EGFR), is associated with aggressive disease and poor prognosis. The EGFR has been proposed as a target for anticancer therapy. Compounds that block ligand-induced EGFR activation have been developed. ZD-1839 (Iressa) is a p.o.-active, quinazoline derivative that selectively inhibits the EGFR tyrosine kinase and is under clinical development in cancer patients. The antiproliferative activity of ZD-1839 alone or in combination with cytotoxic drugs differing in mechanism(s) of action, such as cisplatin, carboplatin, oxaliplatin, paclitaxel, docetaxel, doxorubicin, etoposide, topotecan, and raltitrexed, was evaluated in human ovarian (OVCAR-3), breast (ZR-75-1, MCF-10A ras), and colon cancer (GEO) cells that coexpress EGFR and TGF-alpha. ZD-1839 inhibited colony formation in soft agar in a dose-dependent manner in all cancer cell lines. The antiproliferative effect was mainly cytostatic. However, treatment with higher doses resulted in a 2-4-fold increase in apoptosis. A dose-dependent supra-additive increase in growth inhibition was observed when cancer cells were treated with each cytotoxic drug and ZD-1839. The combined treatment markedly enhanced apoptotic cell death induced by single-agent treatment. [4] Overexpression of the growth factor receptors EGFR and erbB2 occurs frequently in several human cancers and is associated with aggressive tumour behaviour and poor patient prognosis. We have investigated the effects of ZD1839 (Iressa), a novel EGFR tyrosine kinase inhibitor, on the growth, in vitro and in vivo, of human cancer cell lines expressing various levels of EGFR and erbB2. Proliferation of EGFR-overexpressing A431 and MDA-MB-231 cells in vitro was potently inhibited (50%-70%) by ZD1839 with half-maximally effective doses in the low nanomolar range. In parallel, ZD1839 blocked autophosphorylation of EGFR and prevented activation of PLC-gamma 1, ERK MAP kinases and PKB/Akt by EGF. It also inhibited proliferation in EGFR(+) cancer cell lines overexpressing erbB2 (SKBr3, SKOV3, BT474) by between 20% and 80%, effects which correlated with inhibition of EGF-dependent erbB2 phosphorylation and activation of ERK MAP kinase and PKB/Akt in SKOV3 cells.[6] |
| ln Vivo |
Gefitinib (100 mg/kg) enhances radiation therapy's anti-tumor efficaciousness in LoVo tumor xenografts.[3] When established human GEO colon cancer xenografts are given to nude mice bearing these tumors, gefitinib treatment results in a reversible dose-dependent inhibition of tumor growth, as GEO tumors eventually resume the growth rate of controls.[4]
The effect of ZD1839 (‘Iressa’), a specific inhibitor of the tyrosine kinase activity of the epidermal growth factor receptor, on the radiation response of human tumour cells (LoVo colorectal carcinoma) was evaluated in vitro and in vivo. ZD1839 (0.5 μM, incubated days 1–5) significantly increased the anti-proliferative effect of fractionated radiation treatment (2 Gy day−1, days 1–3) on LoVo cells grown in vitro (P=0.002). ZD1839 combined with either single or fractionated radiotherapy in mice bearing LoVo tumour xenografts, also produced a highly significant increase in tumour growth inhibition (P⩽0.001) when compared to treatment with either modality alone. The radio-potentiating effect of ZD1839 was more apparent when radiation was administered in a fractionated protocol. This phenomenon may be attributed to an anti proliferative effect of ZD1839 on tumour cell re-population between radiotherapy fractions. These data suggest radiotherapy with adjuvant ZD1839 could enhance treatment response. Clinical investigation of ZD1839 in combination with radiotherapy is therefore warranted. [3] ZD-1839 treatment of nude mice bearing established human GEO colon cancer xenografts revealed a reversible dose-dependent inhibition of tumor growth because GEO tumors resumed the growth rate of controls at the end of the treatment. In contrast, the combined treatment with a cytotoxic agent, such as topotecan, raltitrexed, or paclitaxel, and ZD-1839 produced tumor growth arrest in all mice. Tumors grew slowly for approximately 4-8 weeks after the end of treatment, when they finally resumed a growth rate similar to controls. GEO tumors reached a size not compatible with normal life in all control mice within 4-6 weeks and in all single agent-treated mice within 6-8 weeks after GEO cell injection. In contrast, 50% of mice treated with ZD-1839 plus topotecan, raltitrexed, or paclitaxel were still alive 10, 12, and 15 weeks after cancer cell injection, respectively. These results demonstrate the antitumor effect of this EGFR-selective tyrosine kinase inhibitor and provide a rationale for its clinical evaluation in combination with cytotoxic drugs. [4] The blockade of epidermal growth factor receptor (EGFR) function with monoclonal antibodies has major antiproliferative effects against human tumors in vivo. Similar antiproliferative effects against some of these same tumors have also been observed with specific inhibitors of the EGFR-associated tyrosine kinase. One such inhibitor, the p.o. active ZD1839 (Iressa), has pronounced antiproliferative activity against human tumor xenografts. We now show that coadministration of ZD1839, as with anti-EGFR, will enhance the efficacy of cytotoxic agents against human vulvar (A431), lung (A549 and SK-LC-16 NSCL and LX-1), and prostate (PC-3 and TSU-PR1) tumors. Oral ZD1839 (five times daily x 2) and cytotoxic agents (i.p. every 3-4 days x 4) were given for a period of 2 weeks to mice with well-established tumors. On this schedule, the maximum tolerated dose (150 mg/kg) of ZD1839 induced partial regression of A431, a tumor that expresses high levels of EGFR, 70-80% inhibition among tumors with low but highly variable levels of EGFR expression (A549, SKLC-16, TSU-PR1, and PC-3), and 50-55% inhibition against the LX-1 tumor, which expresses very low levels of EGFR. ZD1839 was very effective in potentiating most cytotoxic agents in combination treatment against all of these tumors, irrespective of EGFR status, but dose reduction of ZD1839 below its single-agent maximum tolerated dose was required for optimum tolerance. The pronounced growth inhibitory action of the platinums, cisplatin and carboplatinum, as single agents against A431 vulvar, A549 and LX-1 lung, and TSU-PR1 and PC-3 prostate tumors was increased several-fold when ZD1839 was added, with some regression of A431 and PC-3 tumors. Although the taxanes, paclitaxel or docetaxel, as single agents markedly inhibited the growth of A431, LX-1, SK-LC-16, TSU-PR1, and PC-3, when combined with ZD1839, partial or complete regression was usually seen. Against A549, the growth inhibition of doxorubicin was increased 10-fold (>99%) with ZD1839. The folate analogue, edatrexate, was highly growth inhibitory against A549, LX-1, and TSU-PR1, whereas edatrexate combined with ZD1839 resulted in partial or complete regression in these tumors. Against the A431 tumor, paclitaxel alone either was highly growth inhibitory or induced some regression, but when combined with ZD1839, pronounced regression was obtained. Combination with gemcitabine neither added nor detracted from baseline cytotoxic efficacy, whereas ZD1839 combined with vinorelbine was poorly tolerated. Overall, these results suggest that potentiation of cytotoxic treatment with ZD1839 does not require high levels of EGFR expression in the target tumors. They also suggest significant clinical benefit from ZD1839 in combination with a variety of widely used cytotoxic agents [5]. |
| Enzyme Assay |
Gefitinib hydrochloride is an inhibitor with an IC50 value of 2-37 nM in NR6wtEGFR cells that selectively binds to and inhibits the EGFR tyrosine kinase. With an IC50 of 27 nM, gefitinib efficiently prevents PLC-γ phosphorylation in NR6W cells. PLC-γ phosphorylation is low in the NR6wtEGFR and NR6M cell lines, but it is more resistant to gefitinib inhibition in the latter, with IC50 values of 43 nM and 369 nM, respectively. Gefitinib inhibits Akt phosphorylations in the low-EGFR- and EGFRvIII-expressing cell lines, with IC50 values of 220 and 263 nM, respectively.
Crosslinking assay [1] Crosslinking of receptors were carried out as described (Montgomery, 2002). Briefly, Gefitinib-treated cells were washed twice in ice-cold phosphate-buffered saline (PBS) and solubilised in RIPA buffer containing protease and phosphatase inhibitors, 10% glycerol and 1 mM bis(sulfosuccinimidyl) suberate (BS3) for 20 min at 4°C. Glycine at a final concentration of 250 mM was subsequently added for 5 min, followed by centrifugation at 14 000 g for 10 min. Equivalent amounts of protein were resolved by SDS–PAGE and electroblotted onto nitrocellulose membranes. Blotting and antibody incubations were performed as above using anti-EGFR and antiphospho-tyrosine antibodies. |
| Cell Assay |
Exponentially growing cells, such as NR6, NR6M, NR6M, and NR6W cells, are seeded in 96-well plates at a density of 2000 cells/well, allowed to adhere, and then washed in PBS before being incubated overnight in medium containing 0.5% FCS. After that, cells are exposed to different concentrations (0–2 μM) of either gefitinib or the solute controls, DMSO and EGF. Since NR6wtEGFR and NR6W cells can proliferate best at a known EGF concentration, 10 nM and 0.1 nM EGF, respectively, are added to NR6wtEGFR and NR6W cells. NR6 and NR6M cells do not receive additional EGF. An MTT proliferation assay is used to quantify the number of cells after 72 hours.
Proliferation assay [1] Exponentially growing cells were seeded in sextuple in 96-well plates at a concentration of 2000 cells/well, allowed to adhere and subsequently washed in PBS and incubated overnight in medium containing 0.5% FCS. Cells were then treated with varying concentrations of Iressa or the solute control DMSO and EGF. The optimal EGF concentration for inducing proliferation of NR6wtEGFR and NR6W cells has previously been determined and hence NR6wtEGFR and NR6W cells were added 10 and 0.1 nM EGF, respectively (Pedersen et al, unpublished observation). NR6 and NR6M cells were not added EGF. After 72 h the amount of cells were measured by performing a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) proliferation assay. Soft agar assay for anchorage-independent growth [1] Exponentially growing cells (1 × 105) were suspended in 3 ml 0.5% (w/v) NuSieve low-melting agar dissolved in DMEM+0.5% FCS and plated in six-well plates covered with 0.5% agar dissolved in DMEM+0.5% FCS. Cells were then treated with varying concentrations of Iressa or the solute control DMSO. The optimal concentration of EGF for inducing anchorage-independent growth of NR6wtEGFR and NR6W cells has previously been determined and hence NR6wtEGFR were added 10 and 0.1 nM EGF, respectively (Pedersen et al, unpublished observation). NR6 and NR6M were not stimulated with EGF. Cultures in triplicate for each condition were replenished with fresh medium once a week. After 3 weeks the plates were stained with crystal violet and colonies >50 cells were counted. Analysis of proliferation in vitro [6] Cells (5 × 104) were plated in normal growth medium in triplicate into 24-well cell culture clusters. After 24 hr, cells were treated with Gefitinib or DMSO vehicle for a further 48 hr. Cells were then counted using a haemocytometer. Cell viability, assessed by trypan blue staining, was always ≥95% and did not alter with drug treatment. Proliferation was calculated as the increase in cell number during the 48 hr treatment period. Effects of ZD1839 are expressed relative to the increase in cell number observed in control cultures. All experiments were conducted on 3 separate occasions for each cell line. Analysis of apoptosis in vitro [6] Following treatment with Gefitinib, adherent cells were collected by trypsinisation and combined with nonadherent cells. Cells were washed and resuspended in PBS. Cell suspension (50 μl) was applied to a microscope slide using Cytospin 2. Cells were then fixed in paraformaldehyde and treated with Hoechst stain for 2 min. Apoptosis was then quantified by determining the proportion of cells containing nuclei with apoptotic morphology. One hundred cells were assessed in triplicate for each treatment. |
| Animal Protocol |
Female nude mice (cba nu/nu) aged 8–10 weeks are intra-dermal injected with LoVo cells.
100 mg/kg Once daily by oral administration (0.1 mL/10 g body weight) for 14 days Growth of tumours in athymic mice and drug treatments [6] MDA-MB-231 or SKOV3 cells (2 × 106) in a 200 μl solution of 10% FCS with 50% (v/v) Matrigel were injected s.c. into each flank (2 tumours/mouse) of each mouse (n = 15) and allowed to form tumours over a period of 21 days. Mice were then gavaged daily with 75 mg/kg Gefitinib/ZD1839 or vehicle for 14 days. Tumour diameters were caliper-measured twice a week and tumour volume was calculated from the following formula: tumour volume = (width)2 × length/2. At the end of the experiment, tumours were removed from the mice, divided and processed for immunohistochemistry or stored in liquid nitrogen. The growth-inhibitory effect of Gefitinib/ZD1839 was calculated from the following formula: equation image, where d is final tumour volume after ZD1839 treatment, c is tumour volume before ZD1839 treatment, b is final tumour volume after control treatment and a is tumour volume before control treatment. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
Absorbed slowly after oral administration with a mean bioavailability of 60%. Peak plasma levels occurs 3-7 hours post-administration. Food does not affect the bioavailability of gefitinib. Elimination is by metabolism (primarily CYP3A4) and excretion in feces. Excretion is predominantly via the feces (86%), with renal elimination of drug and metabolites accounting for less than 4% of the administered dose. 1400 L [IV administration] 595 mL/min [IV administration] Metabolism / Metabolites Primarily hepatic via CYP3A4. Three sites of biotransformation have been identified: metabolism of the N-propoxymorpholino-group, demethylation of the methoxy-substituent on the quinazoline, and oxidative defluorination of the halogenated phenyl group. Gefitinib has known human metabolites that include O-Desmethyl Gefitinib and 4-Defluoro-4-hydroxy Gefitinib. Biological Half-Life 48 hours [IV administration] |
| Toxicity/Toxicokinetics |
Hepatotoxicity
In large early clinical trials, elevations in serum aminotransferase levels occurred in 9% to 13% of patients treated with standard doses of gefitinib, and 2% to 4% of patients had to stop therapy because of elevations above 5 times the upper limit of normal. Serum enzyme elevations typically arise after 4 to 12 weeks of treatment with a hepatocellular pattern. Immunoallergic and autoimmune features have not been described, but rash is common in patients receiving gefitinib. Most cases of liver injury due to gefitinib in the literature have been minimally or not symptomatic, and the injury resolved within 1 to 2 months of stopping the drug. Restarting therapy was usually but not always followed by rapid recurrence of serum enzyme elevations, and corticosteroid therapy did not appear to prevent this recurrence. In some instances, lower doses were tolerated with minimal or no ALT elevations. Periodic monitoring of liver tests during therapy is recommended. Despite the frequency of serum aminotransferase elevations during gefitinib therapy, cases of clinically apparent liver injury with jaundice are rare. Cases of severe and fatal hepatotoxicity have been reported to the sponsor and monitoring of liver tests during therapy is recommended. Likelihood score: B (likely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of gefitinib during breastfeeding. Because gefitinib is 90% bound to plasma proteins, the amount in milk is likely to be low. However, its half-life is about 48 hours and it might accumulate in the infant. The manufacturer recommends that breastfeeding be discontinued during gefitinib therapy. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding 90% primarily to serum albumin and alpha 1-acid glycoproteins (independent of drug concentrations). |
| References | |
| Additional Infomation |
Gefitinib is a member of the class of quinazolines that is quinazoline which is substituted by a (3-chloro-4-fluorophenyl)nitrilo group, 3-(morpholin-4-yl)propoxy group and a methoxy group at positions 4,6 and 7, respectively. An EGFR kinase inhibitor used for the treatment of non-small cell lung cancer. It has a role as an epidermal growth factor receptor antagonist and an antineoplastic agent. It is an aromatic ether, a member of monochlorobenzenes, a member of monofluorobenzenes, a secondary amino compound, a tertiary amino compound, a member of quinazolines and a member of morpholines.
Gefitinib (originally coded ZD1839) is a drug used in the treatment of certain types of cancer. Acting in a similar manner to erlotinib (marketed as Tarceva), gefitinib selectively targets the mutant proteins in malignant cells. It is marketed by AstraZeneca under the trade name Iressa. Gefitinib is a Kinase Inhibitor. The mechanism of action of gefitinib is as a Protein Kinase Inhibitor. Gefitinib is a selective tyrosine kinase receptor inhibitor used in the therapy of non-small cell lung cancer. Gefitinib therapy is associated with transient elevations in serum aminotransferase levels and rare instances of clinically apparent acute liver injury. Gefitinib has been reported in Penicillium brocae with data available. Gefitinib is an anilinoquinazoline with antineoplastic activity. Gefitinib inhibits the catalytic activity of numerous tyrosine kinases including the epidermal growth factor receptor (EGFR), which may result in inhibition of tyrosine kinase-dependent tumor growth. Specifically, this agent competes with the binding of ATP to the tyrosine kinase domain of EGFR, thereby inhibiting receptor autophosphorylation and resulting in inhibition of signal transduction. Gefitinib may also induce cell cycle arrest and inhibit angiogenesis. (NCI04) A selective tyrosine kinase inhibitor for the EPIDERMAL GROWTH FACTOR RECEPTOR (EGFR) that is used for the treatment of locally advanced or metastatic NON-SMALL CELL LUNG CANCER. Drug Indication For the continued treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of either platinum-based or docetaxel chemotherapies. FDA Label Gefitinib Mylan is indicated as monotherapy for the treatment of adult patients with locally advanced or metastatic nonâsmall cell lung cancer (NSCLC) with activating mutations of EGFRâTK. Iressa is indicated for the treatment of adult patients with locally advanced or metastatic non-small-cell lung cancer with activating mutations of epidermal-growth-factor-receptor tyrosine kinase. Mechanism of Action Gefitinib is an inhibitor of the epidermal growth factor receptor (EGFR) tyrosine kinase that binds to the adenosine triphosphate (ATP)-binding site of the enzyme. EGFR is often shown to be overexpressed in certain human carcinoma cells, such as lung and breast cancer cells. Overexpression leads to enhanced activation of the anti-apoptotic Ras signal transduction cascades, subsequently resulting in increased survival of cancer cells and uncontrolled cell proliferation. Gefitinib is the first selective inhibitor of the EGFR tyrosine kinase which is also referred to as Her1 or ErbB-1. By inhibiting EGFR tyrosine kinase, the downstream signaling cascades are also inhibited, resulting in inhibited malignant cell proliferation. Pharmacodynamics Gefitinib inhibits the intracellular phosphorylation of numerous tyrosine kinases associated with transmembrane cell surface receptors, including the tyrosine kinases associated with the epidermal growth factor receptor (EGFR-TK). EGFR is expressed on the cell surface of many normal cells and cancer cells. Epidermal growth factor receptor (EGFR) is frequently amplified and/or mutated in a number of human tumours and abnormal signalling from this receptor is believed to contribute to the malignant phenotype seen in these tumours. Gefitinib is a small molecule inhibitor that specifically binds and inhibits the EGFR tyrosine kinase and has been shown to inhibit the growth, proliferation, survival and invasion of a range of tumour cells overexpressing EGFR. However, clinical response to gefitinib has failed to correlate with EGFR levels and activity, indicating that other molecular mechanisms such as downstream signalling and mutations could be of importance in predicting clinical response. We therefore investigated the effect of the specific EGFR inhibitor gefitinib on the phosphorylation level, signalling and growth of cells expressing the naturally occurring constitutively active EGFR variant EGFRvIII, a low nontransforming level of EGFR and a high transforming level of EGFR. Results show that levels of gefitinib sufficient to suppress EGFR phosphorylations, EGFR-mediated proliferation and EGFR-mediated anchorage-independent growth are not sufficient to inhibit these features in cells expressing EGFRvIII. Furthermore, the data indicate that long-term exposure of EGFRvIII-expressing cells to low concentrations of gefitinib (0.01-0.1 microM) result in increased phosphotyrosine load of the receptor, increased signalling to ERK and stimulation of proliferation and anchorage-independent growth, presumably by inducing EGFRvIII dimerisation. Higher concentrations of gefitinib (1-2 microM), on the other hand, significantly decreased EGFRvIII phosphotyrosine load, EGFRvIII-mediated proliferation and anchorage-independent growth. Further studies are needed to investigate the implications of these important findings in the clinical setting.[1] The epidermal growth factor receptor (EGFR) is commonly overexpressed in many human tumors and provides a new target for anticancer drug development. ZD1839 ("Iressa"), a quinazoline tyrosine kinase inhibitor selective for the EGFR, has shown good activity in preclinical studies and in the early phase of clinical trials. However, because it remains unclear which tumor types are the best targets for treatment with this agent, the molecular characteristics that correlate with tumor sensitivity to ZD1839 have been studied. In a panel of human breast cancer and other epithelial tumor cell lines, HER2-overexpressing tumors were particularly sensitive to ZD1839. Growth inhibition of these tumor cell lines was associated with the dephosphorylation of EGFR, HER2, and HER3, accompanied by the loss of association of HER3 with phosphatidylinositol 3-kinase, and down-regulation of Akt activity. These studies suggest that HER2-overexpressing tumors are particularly susceptible to the inhibition of HER family tyrosine kinase signaling and suggest novel strategies to treat these particularly aggressive tumors.[2] Transforming growth factor alpha (TGF-alpha) is an autocrine growth factor for human cancer. Overexpression of TGF-alpha and its specific receptor, the epidermal growth factor receptor (EGFR), is associated with aggressive disease and poor prognosis. The EGFR has been proposed as a target for anticancer therapy. Compounds that block ligand-induced EGFR activation have been developed. ZD-1839 (Iressa) is a p.o.-active, quinazoline derivative that selectively inhibits the EGFR tyrosine kinase and is under clinical development in cancer patients. The antiproliferative activity of ZD-1839 alone or in combination with cytotoxic drugs differing in mechanism(s) of action, such as cisplatin, carboplatin, oxaliplatin, paclitaxel, docetaxel, doxorubicin, etoposide, topotecan, and raltitrexed, was evaluated in human ovarian (OVCAR-3), breast (ZR-75-1, MCF-10A ras), and colon cancer (GEO) cells that coexpress EGFR and TGF-alpha. ZD-1839 inhibited colony formation in soft agar in a dose-dependent manner in all cancer cell lines. The antiproliferative effect was mainly cytostatic. However, treatment with higher doses resulted in a 2-4-fold increase in apoptosis. A dose-dependent supra-additive increase in growth inhibition was observed when cancer cells were treated with each cytotoxic drug and ZD-1839. The combined treatment markedly enhanced apoptotic cell death induced by single-agent treatment. [4] Overexpression of the growth factor receptors EGFR and erbB2 occurs frequently in several human cancers and is associated with aggressive tumour behaviour and poor patient prognosis. We have investigated the effects of ZD1839 (Iressa), a novel EGFR tyrosine kinase inhibitor, on the growth, in vitro and in vivo, of human cancer cell lines expressing various levels of EGFR and erbB2. Proliferation of EGFR-overexpressing A431 and MDA-MB-231 cells in vitro was potently inhibited (50%-70%) by ZD1839 with half-maximally effective doses in the low nanomolar range. In parallel, ZD1839 blocked autophosphorylation of EGFR and prevented activation of PLC-gamma 1, ERK MAP kinases and PKB/Akt by EGF. It also inhibited proliferation in EGFR(+) cancer cell lines overexpressing erbB2 (SKBr3, SKOV3, BT474) by between 20% and 80%, effects which correlated with inhibition of EGF-dependent erbB2 phosphorylation and activation of ERK MAP kinase and PKB/Akt in SKOV3 cells. Oral administration of ZD1839 inhibited the growth of MDA-MB-231 and SKOV3 tumours, established as xenografts in athymic mice, by 71% and 32%, respectively. Growth inhibition coincided with reduced proliferation but no change in apoptotic index. Collectively, these results show that ZD1839, at the doses studied, is a potent inhibitor of proliferation not only in cells overexpressing EGFR but also in EGFR(+) cells that overexpress erbB2[6]. |
| Molecular Formula |
C22H24CLFN4O3
|
|---|---|
| Molecular Weight |
446.90
|
| Exact Mass |
446.152
|
| Elemental Analysis |
C, 59.13; H, 5.41; Cl, 7.93; F, 4.25; N, 12.54; O, 10.74
|
| CAS # |
184475-35-2
|
| Related CAS # |
184475-35-2;857091-32-8; 184475-56-7; 184475-55-6; 1173976-40-3; 1173976-40-3; 1228664-49-0
|
| PubChem CID |
123631
|
| Appearance |
White solid powder
|
| Density |
1.3±0.1 g/cm3
|
| Boiling Point |
586.8±50.0 °C at 760 mmHg
|
| Melting Point |
119-1200C
|
| Flash Point |
308.7±30.1 °C
|
| Vapour Pressure |
0.0±1.6 mmHg at 25°C
|
| Index of Refraction |
1.621
|
| LogP |
4.11
|
| Hydrogen Bond Donor Count |
1
|
| Hydrogen Bond Acceptor Count |
8
|
| Rotatable Bond Count |
8
|
| Heavy Atom Count |
31
|
| Complexity |
545
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
ClC1=C(C([H])=C([H])C(=C1[H])N([H])C1C2C(=C([H])C(=C(C=2[H])OC([H])([H])C([H])([H])C([H])([H])N2C([H])([H])C([H])([H])OC([H])([H])C2([H])[H])OC([H])([H])[H])N=C([H])N=1)F
|
| InChi Key |
XGALLCVXEZPNRQ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27)
|
| Chemical Name |
N-(3-chloro-4-fluorophenyl)-7-methoxy-6-(3-morpholin-4-ylpropoxy)quinazolin-4-amine
|
| Synonyms |
Gefitinib; ZD-1839; ZD1839; ZD 1839; Brand name: Iressa
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
|
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2376 mL | 11.1882 mL | 22.3764 mL | |
| 5 mM | 0.4475 mL | 2.2376 mL | 4.4753 mL | |
| 10 mM | 0.2238 mL | 1.1188 mL | 2.2376 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03292133 | Active Recruiting |
Drug: EGF816 Drug: Gefitinib |
Lung Cancer | Massachusetts General Hospital | October 31, 2017 | Phase 2 |
| NCT03122717 | Active Recruiting |
Drug: Gefitinib Drug: Osimertinib |
Non-Small Cell Lung Cancer | Dana-Farber Cancer Institute | May 9, 2017 | Phase 1 Phase 2 |
| NCT03758287 | Active Recruiting |
Drug: Gefitinib Drug: CT053PTSA |
Non-small Cell Lung Cancer | Sunshine Lake Pharma Co., Ltd. | November 2016 | Phase 1 Phase 2 |
| NCT03849768 | Active Recruiting |
Drug: Gefitinib Drug: HS-10296 |
Non Small Cell Lung Cancer | Jiangsu Hansoh Pharmaceutical Co., Ltd. |
February 1, 2019 | Phase 3 |
| NCT02856893 | Active Recruiting |
Drug: Gefitinib Drug: Osimertinib |
NSCLC | European Organisation for Research and Treatment of Cancer - EORTC |
October 10, 2017 | Phase 2 |
A, effect of metformin (MET) alone and in combination with gefitinib (GEF) on cell proliferation, on anchorage-independent growth ability of NSCLC cell lines, and on the induction of apoptosis in CALU-3, CALU-3 GEF-R, and H1299 cell lines.Clin Cancer Res.2013 Jul 1;19(13):3508-19. |
Effects on the downstream pathway by combined treatment of metformin and gefitinib. Western blotting of EGFR, MAPK, AKT p70S6K, and S6 activation following treatment with the indicated concentration of metformin and gefitinib in CALU-3 and CALU-3 GEF-R cell lines. β-Actin was included as a loading control.Clin Cancer Res.2013 Jul 1;19(13):3508-19. |
Effects of the combination treatment of metformin and gefitinib on NSCLC tumor xenografts.Clin Cancer Res.2013 Jul 1;19(13):3508-19. |
 |
 |