
| Size | Price | Stock | Qty |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g | |||
| Other Sizes |
Purity: ≥98%
Entinostat (formerly known as MS-275; SNDX-275) is a potent, benzamide-based and class-selective but generally pan-HDAC (histone deacetylase) inhibitor with potential anticancer activity. It is more selective for HDAC1/2/3 over HDACs 4, 6, 8, and 10, and it strongly inhibits HDAC1, HDAC2, and HDAC3 with IC50 values of 0.24, 0.45, and 0.25 μM in cell-free experiments, respectively. By attaching to and inhibiting histone deacetylase, an enzyme that controls chromatin structure and gene transcription, entinostat may have anticancer properties. In human leukemia cells, this agent appears to have dose-dependent effects, including, at low drug concentrations, cyclin-dependent kinase inhibitor 1A (p21/CIP1/WAF1)-dependent growth arrest and differentiation.
| Targets |
HDAC1 ( IC50 = 243 nM ); HDAC3 ( IC50 = 248 nM ); HDAC2 ( IC50 = 453 nM )
|
|
|---|---|---|
| ln Vitro |
|
|
| ln Vivo |
|
|
| Enzyme Assay |
HDAC activity biochemical assays are performed by Nanosyn in 384-well microplates with a reaction volume of 10 μL. Five microliters of a 2× HDAC inhibitor (such as Entinostat), four microliters of 2.5× enzyme, and one microliter of 10× substrate are combined with assay buffer (100 mM HEPES, pH 7.5, 25 mM KCl, 0.1% BSA, 0.01% Triton X-100, 1% DMSO) in a typical enzymatic reaction. In the enzymatic assays, the final concentration of each HDAC ranges from 0.5 to 5 nM. In every experiment, a final substrate concentration of 1 μM FAM-RHKK(Ac)-NH2 or FAM-RHKK(trifluoroacetyl)-NH2 is employed, and it is discovered to be lower than the calculated Km,app for every enzyme[1].
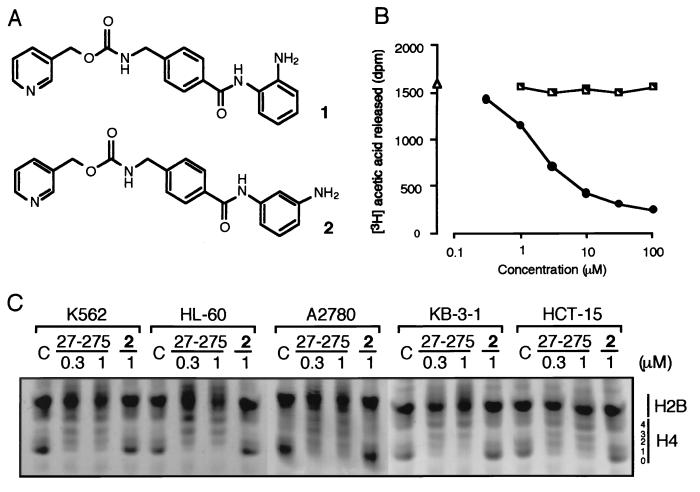
Enzymatic HDAC Activity Assays [1] Biochemical assays of HDAC activity were carried out by Nanosyn in a reaction volume of 10 μl in 384-well microplates. A standard enzymatic reaction contained 5 μl of 2× HDAC inhibitor, 4 μl of 2.5× enzyme, and 1 μl of 10× substrate in assay buffer (100 mm HEPES, pH 7.5, 25 mm KCl, 0.1% BSA, 0.01% Triton X-100, 1% DMSO). Final concentration of all HDACs in the enzymatic assays was between 0.5 and 5 nm. A final substrate concentration of 1 μm FAM-RHKK(Ac)-NH2 or FAM-RHKK(trifluoroacetyl)-NH2 was used in all assays and found to be below the determined Km, app for each enzyme. All inhibitors were serially diluted in DMSO prior to cross-dilution in assay buffer and were incubated with enzyme for 15 min prior to initiating the reaction by the addition of substrate. After incubation for 3 h, the reaction was terminated by the addition of EDTA and SDS to final concentrations of 24 mm and 0.04%, respectively. The product and substrate in each reaction were separated using a 12-sipper microfluidic chip run on a Caliper LC3000®. The separation conditions used a downstream voltage of −800V, an upstream voltage of −3000 V, and a screening pressure of −1.4 p.s.i. The product and substrate fluorescence was excited at 488 nm and detected at 530 nm. Substrate conversion was calculated from the electrophoregram using HTS Well Analyzer software. Assay for Histone Deacetylase. [3] HDA was partially purified as described by Yoshida et al. with slight modifications. K562 cells (2.5 × 108) were disrupted in 15 ml of HDA buffer (15 mM potassium phosphate, pH 7.5/5% glycerol/0.2 mM EDTA). Nuclei of the cells were collected by centrifugation (35,000 × g, 10 min) and were resuspended in 15 ml of HDA buffer containing 1 M (NH4)2SO4. After sonication to reduce viscosity, the supernatant was collected by centrifugation, solid (NH4)2SO4 was added to the supernatant to make the final concentration 3.5 M, and was stirred for 1 h at 0°C. The precipitates collected by centrifugation were dissolved again with 4 ml of HDA buffer and were dialyzed against 2 liters of HDA buffer. The dialysate was loaded onto MonoQ HR5/5 (Amersham Pharmacia) equilibrated with HDA buffer, and the proteins were eluted with a linear gradient of 0–1 M NaCl in 30 ml of HDA buffer. A single peak of HDA activity was eluted at 0.4 M NaCl, and the fraction was stored at −80°C until use. Nuclear histones were labeled by incubation of K562 cells (108 cells) in a 25 ml of growth medium containing 0.5 mCi/ml [3H]sodium acetate (152.8 GBq/mmol; NEN) and 5 mM NaBu at 37°C for 1 h. Histones were extracted as described. HDA-inhibitory activity of the compound was estimated in 50 μl of reaction mixture containing 2 μl of the above HDA fraction, 100 μg/ml of [3H]acetylated histones, and 5 μl of the compound dissolved in HDA buffer at 37°C for 10 min. [3H]acetic acid released by the reaction was extracted with 50 μl of 1M HCl and 0.55 ml of ethyl acetate, and the radioactivity in the solvent layer was measured by liquid-scintillation counting. To assess in vivo HDA inhibition, cellular histones were extracted and examined by acid/urea/Triton X-100 PAGE followed by staining with Coomasie brilliant blue R-250, as described. |
|
| Cell Assay |
SH-SY5Y cells are split twice a week and kept in a humidified incubator with 5% CO2 at 37°C under standard culture conditions. After plating cells at a density of 2500 cells per well in a 20-μL volume of DMEM/F-12 culture media supplemented with 10% FBS, the cells are left to adhere for the entire night in black 384-well plates. After being serially diluted in 100% DMSO the next day, HDAC inhibitors (such as Entinostat) are then cross-diluted into culture media. To achieve the desired inhibitor final concentration (e.g., 0.1% DMSO), 5 μL of the compound (e.g., Entinostat) diluted in media is added to the appropriate well of the cell plate. Cellular ATP levels are quantified using CellTiter-Glo reagents after treated cells are incubated for 6, 24, 48, 72, or 96 hours under standard tissue culture conditions. Similarly, media from different cell plates are aspirated after 6 hours of incubation with HDAC inhibitors (such as Entinostat), and cells are once again washed with media free of inhibitors. After 24, 48, 72, or 96 hours of incubation, the cells are given 25 μL of media supplemented with 10% FBS and 0.1% DMSO (no inhibitors), and the levels of cellular ATP are measured using CellTiter-Glo. An Envision Instrument with a 0.1 s count time is used to measure luminosity at each time point[1].
|
|
| Animal Protocol |
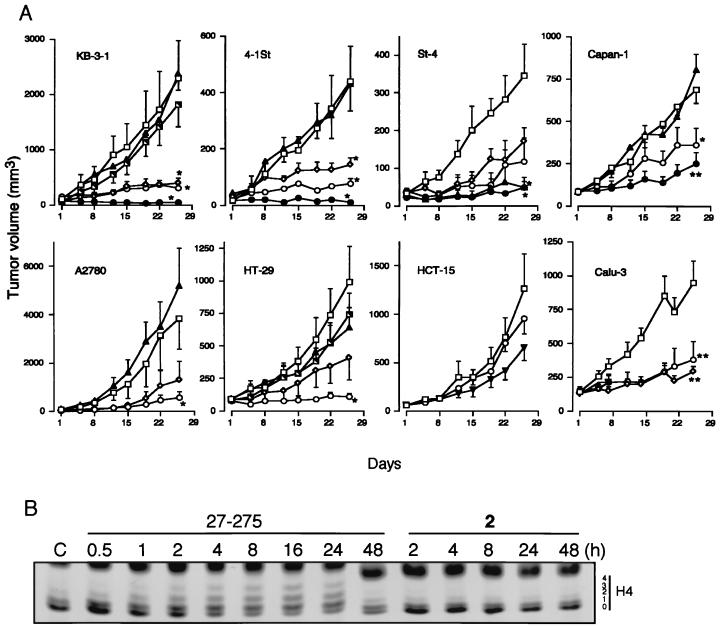
Mice: Subcutaneous injection of A2780 cells (9×106) in PBS suspension is administered subcutaneously into the flank of a naked mouse. For the remaining tumor lines, KB-3-1, HCT-15, 4-1St, Calu-3, St-4, Capan-1, and HT-29, the tumors are passaged multiple times prior to initiating in vivo antitumor testing. A trocar needle is used to implant a tumor lump, measuring 2-3 mm in diameter, subcutaneously into the flank of a nude mouse. Once the tumors are confirmed to have grown in the body (tumor size, 20-100 mm3), treatment with the drugs is initiated in four or five mice per experimental group. For four weeks, one oral dose of entinostat is given five days a week. Tumor width and length are measured twice a week, and the volume of the tumor is computed.
Rats: Male Lewis rats (weight: 170-200 g, 8-10 weeks) are kept in a 12-hour light/dark cycle with unrestricted access to food and drink. Six rats per group receive an intraperitoneal injection of EntinostatMS-275 (3.5 mg/kg) every day from day 10 to day 14 as part of a therapeutic treatment. EntinostatMS-275 is dissolved in phosphate buffered saline (PBS) for injection, and control rats are administered the same volume (1 mL) of PBS. In Vivo Antitumor Activity. [3] A2780 cells (9 × 106) grown in vitro were suspended in PBS and were injected subcutaneously into the flank of nude mouse. For the other tumor lines, KB-3-1, HCT-15, 4-1St, Calu-3, St-4, Capan-1, and HT-29, tumors were passaged several times before starting in vivo antitumor testing, and a tumor lump (2–3 mm in diameter) was transplanted subcutaneously into the flank of a nude mouse by using a trocar needle. Treatment (four or five mice in each experimental group) with the drugs was started after the tumors were confirmed to have grown in the body (tumor size, 20–100 mm3). Entinostat/MS-27-275 and compound 2, both dissolved with 0.05 N HCl, 0.1% Tween 80, and 5-fluorouracil (5-FU) and diluted with physiological saline, were administered orally once daily 5 days per week for 4 weeks. Tumor length and width were monitored twice weekly, and tumor volume was calculated as described. EAN induction and Entinostat/MS-275 treatment [4] EAN was induced as described (Zhang et al., 2008a). Briefly, rats were immunized by s.c. injection at the basal part of tails with 100 μL of an inoculum containing 100 μg of synthetic neuritogenic P2 peptide 57–81. Neurological scores of EAN were evaluated every day as follows: 0=normal, 1=reduced tonus of tail, 2=limp tail, impaired righting, 3=absent righting, 4=gait ataxia, 5=mild paresis of the hind limbs, 6=moderate paraparesis, 7=severe paraparesis or paraplegia of the hind limbs, 8=tetraparesis, 9=moribund, and 10=death. For therapeutic treatment, EAN rats received i.p. injection of Entinostat/MS-275 (3.5 mg/kg) daily from day 10 to day 14 (six rats/group). For injection, EntinostatMS-275 was suspended in phosphate buffered saline (PBS) and the same volume (1 ml) of PBS was given to control rats. |
|
| ADME/Pharmacokinetics |
A summary of the pharmacokinetics of MS-275 is presented in Table 5. In part 1, with every other week dosing, the maximum plasma concentrations (Cmax) and the early part of the AUC were poorly characterized, because the first sampling point was taken after tmax. A plasma concentration × time curve following single doses of 2, 4, and 6 mg/m2 is shown in Fig. 1.
On all dosing schedules, maximum plasma levels of MS-275 were reached within 1 h after single and repeated dose administration. Mean Cmax after the first administration increased almost dose-proportionally. MS-275 plasma concentrations declined to ~4% of Cmax within 4 h after single administration in parts 2 and 3 of the study, where the Cmax was well defined, indicating a rapid distribution into tissues. This initial distribution phase was followed by a slower secondary disposition phase. However, terminal half-life values could not be determined appropriately in most patients, because the perceivable linear part of the curve was <2 half-lives. Thus, the calculated terminal half-life values, as well as AUCand apparent clearance values, were only estimated and may be further considered as approximate reference values. The terminal half-life values were estimated to range between 60 and 150 h, irrespective of the dose or schedule, when the perceivable linear part of the curve could be followed for a relatively long time (up to ~168 h post-dose). The accumulation of the drug in plasma following once-weekly and twice-weekly dosing schedules was evaluated to a limited extent due to deviations from the dosing schedule such as skipped doses and dose reductions due to toxicity. Based on the limited data, plasma drug concentrations appeared to increase continuously up to the last dose in cycle 1, indicating that steady state was not achieved in the twice-weekly treatment cohort. There was no clear indication of drug accumulation following once-weekly treatment. https://pubmed.ncbi.nlm.nih.gov/18579665/ |
|
| Toxicity/Toxicokinetics |
Hematologic toxicity and laboratory abnormalities [https://pubmed.ncbi.nlm.nih.gov/18579665/]
The numbers of cycles with hematologic toxicities are listed in Table 3. Overall, hematologic toxicities were rare and none was dose limiting. Grade 3 hemoglobin occurred in 5 of 149 cycles (3%): 1 on the 2 mg/m2 twice-weekly schedule and 4 in the 5 mg/m2 weekly cohort. Grade 3 or 4 neutropenia was seen in 6 of 149 cycles (4%): 2 at 2 mg/m2 and 1 at 4 mg/m2 every other week and in 3 on the 5 mg/m2 weekly schedule. No grade 3 or 4 thrombocytopenia was observed. There was no relationship between the development of myelosuppression and prior chemotherapy or radiation therapy, and none of the episodes was complicated by fever, serious infection, or bleeding. Nonhematologic toxicity [https://pubmed.ncbi.nlm.nih.gov/18579665/] Nonhematologic toxicity is shown in Table 4. The most common adverse events were nausea and asthenia [occurring in 48 and 47 of 149 cycles (32%), respectively]. The second most common event, anorexia, occurred in 23 of 149 cycles (15%). The most common grade 3 or 4 event was asthenia, occurring in 7 of 149 cycles (5%). Asymptomatic hypophosphatemia was common and constituted DLT in two patients. The hypophosphatemia was not associated with other toxicities such as renal insufficiency or other electrolyte imbalances, and no serious complications were observed. One patient with metastatic colon cancer also experienced concurrent grade 3 hyponatremia during an episode of massive abdominal ascites and dehydration associated with rapid tumor progression and decreased oral intake. This patient had no prior history of renal disease, electrolyte wasting, or prior chemotherapy treatment known to contribute to electrolyte wasting. This patient's dehydration and electrolyte abnormalities resolved within 72 h of therapeutic paracentesis, i.v. fluid, and colloid administration. Patients who had grade ≥2 hypophosphatemia underwent analysis of urine and serum electrolytes, were given daily oral phosphorus supplements, and were monitored at least weekly until stabilization of serum phosphorus levels to within normal range. With other HDAC inhibitors, including the hydroxamic acid derivatives, there has been concern for cardiac rhythm disturbances and myocardial infarction, including prolonged QTc intervals and T- and ST-wave abnormalities, particularly based preclinical models. In this study, electrocardiograms were required at baseline, through cycle 1, at the beginning of cycle 2, and at the end of study participation for the every other week dosing and at baseline and as clinically indicated on the twice-weekly and weekly schedules. MUGA scans were done at baseline, before cycles 2 and 4, and every 6 weeks after cycle 4 on the every other week schedule and at baseline and as clinically indicated in the twice-weekly and weekly treatment groups. Eighteen patients had electrocardiograms after the baseline evaluation: 10 on every other week dosing, 3 on twice-weekly dosing, and 5 on weekly dosing schedules. Ten patients completed serial MUGA scanning: 6 on every other week dosing and 2 each on twice-weekly and weekly dosing schedules. No significant electrocardiograms or MUGA abnormalities attributed at least possibly related to MS-275 were noted. |
|
| References |
|
|
| Additional Infomation |
Entinostat is a member of the class of benzamides resulting from the formal condensation of the carboxy group of the pyridin-3-ylmethyl carbamate derivative of p-(aminomethyl)benzoic acid with one of the amino groups of benzene-1,2-diamine. It is an inhibitor of histone deacetylase isoform 1 (HDAC1) and isoform 3 (HDAC3). It has a role as an EC 3.5.1.98 (histone deacetylase) inhibitor, an antineoplastic agent and an apoptosis inducer. It is a member of pyridines, a carbamate ester, a substituted aniline, a primary amino compound and a member of benzamides. It is functionally related to a 1,2-phenylenediamine.
Entinostat is under investigation for the treatment and other of Volunteers, Breast Cancer, Human Volunteers, and Normal Volunteers. Entinostat has been investigated for the treatment of Non-Small Lung Cancer, Epigenetic Therapy. Entinostat is a synthetic benzamide derivative with potential antineoplastic activity. Entinostat binds to and inhibits histone deacetylase, an enzyme that regulates chromatin structure and gene transcription. This agent appears to exert dose-dependent effects in human leukemia cells including cyclin-dependent kinase inhibitor 1A (p21/CIP1/WAF1)-dependent growth arrest and differentiation at low drug concentrations; a marked induction of reactive oxygen species (ROS); mitochondrial damage; caspase activation; and, at higher concentrations, apoptosis. In normal cells, cyclin-dependent kinase inhibitor 1A expression has been associated with cell-cycle exit and differentiation. Effects of the histone deacetylase (HDAC) inhibitor MS-275 have been examined in human leukemia and lymphoma cells (U937, HL-60, K562, and Jurkat) as well as in primary acute myelogenous leukemia blasts in relation to differentiation and apoptosis. MS-275 displayed dose-dependent effects in each of the cell lines. When administered at a low concentration (e.g., 1 micro M), MS-275 exhibited potent antiproliferative activity, inducing p21(CIP1/WAF1)-mediated growth arrest and expression of differentiation markers (CD11b) in U937 cells. These events were accompanied by an increase in hypophosphorylated retinoblastoma protein and down-regulation of cell cycle-related proteins including cyclin D1. However, at higher concentrations (e.g., 5 micro M), MS-275 potently induced cell death, triggering apoptosis in approximately 70% of cells at 48 h. In contrast to other HDAC inhibitors such as apicidin, the extrinsic, receptor-mediated pathway played a minimal role in MS-275 lethality. However, MS-275 potently induced a very early (e.g., within 2 h) increase in reactive oxygen species (ROS), followed by the loss of mitochondrial membrane potential (Delta psi(m)) and cytosolic release of cytochrome c. These events culminated in activation of the caspase cascade, manifested by poly(ADP-ribose) polymerase, p21(CIP1/WAF1), p27(KIP), Bcl-2, and retinoblastoma protein degradation. MS-275 exposure also resulted in diminished expression of cyclin D1 and the antiapoptotic proteins Mcl-1 and XIAP. Administration of the free radical scavenger L-N-acetylcysteine blocked MS-275-mediated mitochondrial injury and apoptosis, suggesting a primary role for ROS generation in MS-275-associated lethality. Lastly, U937 cells stably expressing a p21(CIP1/WAF1) antisense construct were significantly more sensitive to MS-275-mediated apoptosis than controls, but they were impaired in their differentiation response. Together, these findings demonstrate that MS-275 exerts dose-dependent effects in human leukemia cells, i.e., p21(CIP1/WAF1)-dependent growth arrest and differentiation at low drug concentrations and a marked induction of ROS, mitochondrial damage, caspase activation, and apoptosis at higher concentrations.[2] Synthetic benzamide derivatives were investigated for their ability to inhibit histone deacetylase (HDA). In this study, one of the most active benzamide derivatives, MS-27-275, was examined with regard to its biological properties and antitumor efficacy. MS-27-275 inhibited partially purified human HDA and caused hyperacetylation of nuclear histones in various tumor cell lines. It behaved in a manner similar to other HDA inhibitors, such as sodium butyrate and trichostatin A; MS-27-275 induced p21(WAF1/CIP1) and gelsolin and changed the cell cycle distribution, decrease of S-phase cells, and increase of G1-phase cells. The in vitro sensitivity spectrum of MS-27-275 against various human tumor cell lines showed a pattern different than that of a commonly used antitumor agent, 5-fluorouracil, and, of interest, the accumulation of p21(WAF1/CIP1) tended to be faster and greater in the cell lines sensitive to MS-27-275. MS-27-275 administered orally strongly inhibited the growth in seven of eight tumor lines implanted into nude mice, although most of these did not respond to 5-fluorouracil. A structurally analogous compound to MS-27-275 without HDA-inhibiting activity showed neither the biological effects in cell culture nor the in vivo therapeutic efficacy. These results suggest that MS-27-275 acts as an antitumor agent through HDA inhibition and may provide a novel chemotherapeutic strategy for cancers insensitive to traditional antitumor agents.[3] |
| Molecular Formula |
C21H20N4O3
|
|
|---|---|---|
| Molecular Weight |
376.41
|
|
| Exact Mass |
376.153
|
|
| Elemental Analysis |
C, 67.01; H, 5.36; N, 14.88; O, 12.75
|
|
| CAS # |
209783-80-2
|
|
| Related CAS # |
|
|
| PubChem CID |
4261
|
|
| Appearance |
White off white solid powder
|
|
| Density |
1.3±0.1 g/cm3
|
|
| Boiling Point |
566.7±50.0 °C at 760 mmHg
|
|
| Melting Point |
159-160ºC
|
|
| Flash Point |
296.6±30.1 °C
|
|
| Vapour Pressure |
0.0±1.6 mmHg at 25°C
|
|
| Index of Refraction |
1.672
|
|
| LogP |
1.46
|
|
| Hydrogen Bond Donor Count |
3
|
|
| Hydrogen Bond Acceptor Count |
5
|
|
| Rotatable Bond Count |
7
|
|
| Heavy Atom Count |
28
|
|
| Complexity |
508
|
|
| Defined Atom Stereocenter Count |
0
|
|
| SMILES |
O=C(NCC1=CC=C(C=C1)C(NC2=CC=CC=C2N)=O)OCC3=CC=CN=C3
|
|
| InChi Key |
INVTYAOGFAGBOE-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26)
|
|
| Chemical Name |
pyridin-3-ylmethyl N-[[4-[(2-aminophenyl)carbamoyl]phenyl]methyl]carbamate
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.64 mM) (saturation unknown) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.08 mg/mL (5.53 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 2.08 mg/mL (5.53 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 4: ≥ 2.08 mg/mL (5.53 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 20.8 mg/mL clear DMSO stock solution to 900 μL corn oil and mix evenly. Solubility in Formulation 5: 2% DMSO+30% PEG 300: 10mg/mL Solubility in Formulation 6: 3% DMSO + 22% Castor oil + 75% Saline |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6567 mL | 13.2834 mL | 26.5668 mL | |
| 5 mM | 0.5313 mL | 2.6567 mL | 5.3134 mL | |
| 10 mM | 0.2657 mL | 1.3283 mL | 2.6567 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02569320 | Active Recruiting |
Drug: Entinostat Drug: Nivolumab |
Renal Cell Carcinoma | Roberto Pili | August 31, 2018 | Phase 2 |
| NCT02936752 | Active Recruiting |
Drug: Entinostat Biological: Pembrolizumab |
Myelodysplastic Syndrome | National Cancer Institute (NCI) |
April 3, 2017 | Phase 1 |
| NCT03501381 | Active Recruiting |
Drug: Entinostat Drug: Interleukin-2 |
Renal Cell Carcinoma | Roberto Pili | May 24, 2018 | Phase 2 |
| NCT03978624 | Active Recruiting |
Drug: Pembrolizumab Drug: Entinost |
Bladder Cancer | UNC Lineberger Comprehensive Cancer Center |
September 23, 2020 | Phase 2 |
| NCT03280563 | Active Recruiting |
Drug: Entinostat Drug: Exemestane |
Breast Neoplasms | Hoffmann-La Roche | December 26, 2017 | Phase 1 Phase 2 |
|
|---|
 |
 |