
| Size | Price | Stock | Qty |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| Other Sizes |
| Targets |
p110δ (IC50 = 2.5 nM); p110γ (IC50 = 27.4 nM); p110β (IC50 = 85 nM); p110α (IC50 = 1602 nM)
|
|---|---|
| ln Vitro |
IPI-145 inhibits human T-cell proliferation with an EC50 of 9.5 nM and suppresses murine/human B-cell proliferation with an EC50 of 0.5 nM/0.5 nM.[1]
|
| ln Vivo |
IPI-145 (10 mg/kg, p.o.) exhibits good pharmacokinetics in mice and rats, with Cmax and AUC values of 390 ng/mL and 137 ng•h/mL, respectively. With a 50% ear swelling in the murine DTH model, IPI-145 (10 mg/kg) is effective. In a rat collagen-induced arthritis (CIA) model, IPI-145 (10 mg/kg) exhibits a dose-dependent effect. In the rat CIA model, IPI-145 reduces inflammation and safeguards joint bone and cartilage. In a model of adjuvant-induced polyarthritis in rats, IPI-145 (10 mg/kg,QD) exhibits activity. [1]
|
| Enzyme Assay |
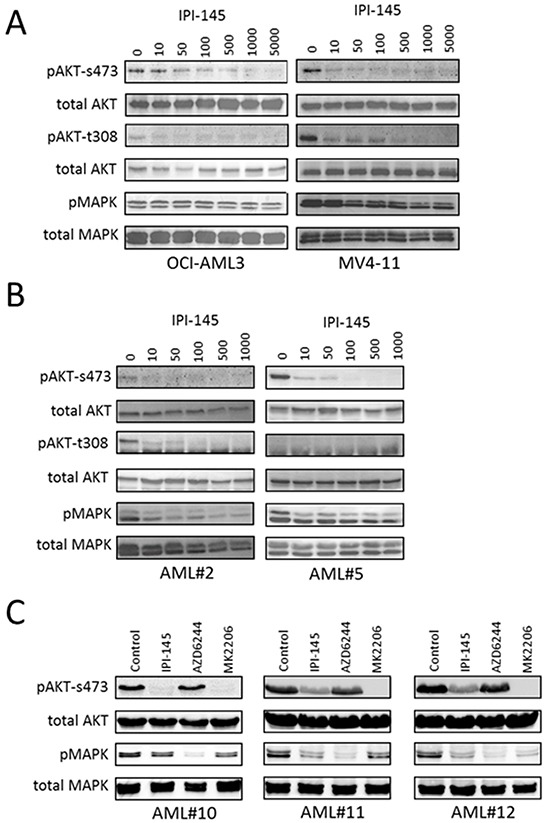
Duvelisib is a selectivitep100δinhibitor with IC50of 2.5 nM, 27.4 nM, 85 nM and 1602 nM for p110δ, P110γ, p110β and p110α, respectively.PI3Kδ and PI3Kγ inhibition with IPI-145 has anti-proliferative activity in primary AML cells by inhibiting the activity of AKT and MAPK. Pre-treatment of AML cells with IPI-145 inhibits both adhesion and migration of AML blasts to bone marrow stromal cells.
|
| Cell Assay |
IPI-145 (10 μM) was applied to AML cell lines, and the cells were then cultured for 72 hours.
|
| Animal Protocol |
Brown Norway rats
(0.1, 0.3, 1, or 10 mg/kg p.o. |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
Duvelisib is rapidly absorbed and its peak plasma concentration is reached 1-2 hours after initial administration with a bioavailability of 42% and with a minimal accumulation whose rate ranges between 1.5 and 2.9. The maximal plasma concentration is reported to range in between 471 to 3294 ng/ml with a systemic exposure ranging from 2001 to 19059 ng.h/ml. Changes in the administered dose produce correspondent changes in all absorption parameters indicating a dose-response profile. Duvelisib is eliminated after 3.5-9.5 hours when administered as a single dose and after 6.5-11.7 hours when given in multiple doses. From the administered dose, 79% os excreted in feces and 14% in urine. About 10% of the total administered dose is secreted unchanged. The volume of distribution of duvelisib ranges from 26 to 102 L. Duvelisib clearance rate is reported to be in the range of 3.6 to 11.2 L/h. Metabolism / Metabolites Duvelesib is mainly metabolized by CYP3A4. Biological Half-Life The reported half-life of duvelisib is in the range of 5.2 to 10.9 hours. |
| Toxicity/Toxicokinetics |
Hepatotoxicity
In clinical trials of duvelisib in patients with CLL and lymphoma, the rates of serum enzyme elevations during therapy ranged from 39% to 57% and were above 5 times the ULN in 3% to 8%. Serum enzyme elevations typically arose within 4 to 12 weeks of starting therapy and usually resolved rapidly with dose reduction or temporary discontinuation. In many instances, the serum aminotransferase elevations resolved spontaneously and most (but not all) patients were able to restart duvelisib without recurrence. While there were no reported cases of clinically apparent liver injury with jaundice, up to 35% of patients discontinued duvelisib because of serum enzyme elevations and all patients were followed carefully during treatment. In one study, 2% of treated patients developed concurrent elevations in serum aminotransferase and bilirubin levels but there were no episodes considered to be clinically apparent liver injury and no deaths due to liver failure. Duvelisib has not been widely used since its approval, and its potential for causing acute clinically apparent liver injury with jaundice has not been well defined. Because duvelisib affects B cell function, it may also be capable of inducing reactivation of hepatitis B, although in published trials of the agent, reactivation was not reported. Likelihood score: E* (unproved but suspected cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of duvelisib during breastfeeding. Because duvelisib is 98% bound to plasma proteins, the amount in milk is likely to be low. However, because of its potential toxicity in the breastfed infant, the manufacturer recommends that breastfeeding be discontinued during duvelisib therapy and for at least 1 month after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding The protein binding of duvelisib is greater than 98% and this level is not dependent on serum concentration. It is reported that duvelisib is a substrate of P-gp and BCRP. |
| References | |
| Additional Infomation |
Pharmacodynamics
Preclinical data showed that duvelisib presents cytotoxic actions at micromolar doses and antagonizes the activation of downstream signaling even in the presence of the mutation BTK C481S, which allows for the treatment of patients resistant to ibrutinib. In clinical trials, duvelisib was compared to ofatumumab in patients with chronic lymphocytic leukemia or small lymphocytic leukemia. This trials reported a median progression-free survival of 16.4 months and an overall response rate of 78% which were almost 2-fold what it was reported for ofatumumab. In clinical trials of follicular lymphoma, duvelisib presented and overall response rate of 42% from which almost all the patients observed a partial response. Of the responding patients, 43% maintained the response for at least 6 months and 17% for at least 12 months. |
| Molecular Formula |
C22H17CLN6O
|
|---|---|
| Molecular Weight |
416.86
|
| Exact Mass |
416.115
|
| Elemental Analysis |
C, 63.39; H, 4.11; Cl, 8.50; N, 20.16; O, 3.84
|
| CAS # |
1201438-56-3
|
| Related CAS # |
Duvelisib (R enantiomer);1261590-48-0
|
| PubChem CID |
50905713
|
| Appearance |
white solid powder
|
| Density |
1.5±0.1 g/cm3
|
| Melting Point |
>190 ºC
|
| Index of Refraction |
1.757
|
| LogP |
4.6
|
| Hydrogen Bond Donor Count |
2
|
| Hydrogen Bond Acceptor Count |
5
|
| Rotatable Bond Count |
4
|
| Heavy Atom Count |
30
|
| Complexity |
668
|
| Defined Atom Stereocenter Count |
1
|
| SMILES |
ClC1=C([H])C([H])=C([H])C2=C1C(N(C1C([H])=C([H])C([H])=C([H])C=1[H])C(=C2[H])[C@]([H])(C([H])([H])[H])N([H])C1C2=C(N=C([H])N=1)N=C([H])N2[H])=O
|
| InChi Key |
SJVQHLPISAIATJ-ZDUSSCGKSA-N
|
| InChi Code |
InChI=1S/C22H17ClN6O/c1-13(28-21-19-20(25-11-24-19)26-12-27-21)17-10-14-6-5-9-16(23)18(14)22(30)29(17)15-7-3-2-4-8-15/h2-13H,1H3,(H2,24,25,26,27,28)/t13-/m0/s1
|
| Chemical Name |
(S)-3-(1-((9H-purin-6-yl)amino)ethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one
|
| Synonyms |
IPI145; IPI 145; IPI-145; INK1197; INK 1197; INK-1197; Duvelisib; trade name: Copiktra
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|
|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 2.5 mg/mL (6.00 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 2.5 mg/mL (6.00 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 25.0 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: 30% PEG400+0.5% Tween80+5% Propylene glycol : 30mg/mL |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3989 mL | 11.9944 mL | 23.9889 mL | |
| 5 mM | 0.4798 mL | 2.3989 mL | 4.7978 mL | |
| 10 mM | 0.2399 mL | 1.1994 mL | 2.3989 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
A Study of Ruxolitinib and Duvelisib in People With Lymphoma
CTID: NCT05010005
Phase: Phase 1 Status: Active, not recruiting
Date: 2024-08-23
|
 |
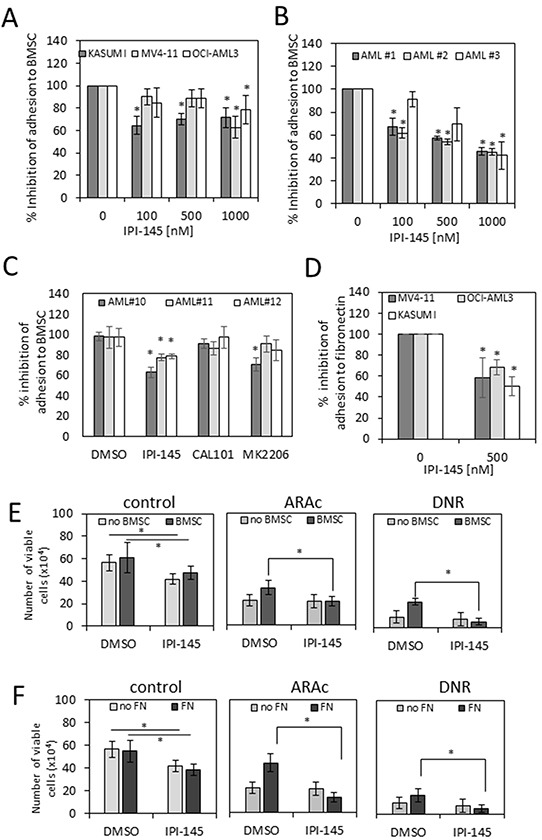 |
Targeting PI3K inhibits AML survival in AML cell lines and primary AML blasts. |
IPI-145 inhibits AKT phosphorylation in AML. Oncotarget. 2016 Jun 28;7(26):39784-39795. |
IPI-145 inhibits adhesion of AML blasts to primary BMSC. Oncotarget. 2016 Jun 28;7(26):39784-39795. |