
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
Purity: ≥98%
Crenolanib (formerly also known as CP868596; RO-002; ARO-002), a benzimidazole compound, is an orally bioavailable and selective small molecule inhibitor of platelet-derived growth factor receptor (PDGFRα/β) with potential anticancer activity. In CHO cells, it inhibits PDGFRα/β with Kd values of 2.1 nM/3.2 nM. In clinical trials, the AROG company is developing an investigational medication called crenolanib to treat a variety of cancers, including gliomas, gastrointestinal stromal tumors, and acute myeloid leukemia. By attaching itself to PDGFR and blocking it, clenolanib inhibits tumor angiogenesis and tumor cell proliferation. This can also lead to the inhibition of PDGFR-related signal transduction pathways.
| Targets |
PDGFRα (Kd = 2.1 nM); PDGFRβ (Kd = 3.2 nM); FLT3 (Kd = 0.74 nM)
|
|
|---|---|---|
| ln Vitro |
|
|
| ln Vivo |
|
|
| Enzyme Assay |
The WST-1 assay is used to quantify the amount of viable cells that remain after drug therapy. In summary, 100 μL complete medium is used to seed 1×103 cells per well in 96-well tissue culture plates. The cells are then incubated with crenolanib (0-10 μM) at 37°C in 5% CO2 for 96 hours. Each well is then filled with 10 μL of WST-1 reagent, incubated for an additional two hours, and the color developed is measured in accordance with the manufacturer's instructions. Three duplicates of each experiment are run. Using GraphPad Prism V software, IC50 concentrations are determined by utilizing the least square fit of dose-response inhibition in a non-linear regression model.
|
|
| Cell Assay |
Chinese hamster ovary (CHO) cells are exposed to different doses of Crenolanib after being transiently transfected with mutant or wild type PDGFRα constructs. According to guidelines, recombinant DNA experiments are carried out under biosafety level 2 conditions. Prepared protein lysates from cell lines are immunoprecipitated with anti-PDGFRα antibodies, and then PDGFRα is sequentially immunoblotted. Photoshop software is used to perform densitometry, which normalizes the level of phosphor-PDGFRα to total protein in order to quantify the drug effect. The IC50 values are calculated mathematically using Calcusyn 2.1 software by analyzing the results of proliferation and densitometry experiments. For each mutation, the IC50 values of Crenolanib are compared using the Wilcoxon Rank Sum Test.
|
|
| Animal Protocol |
A549 cells are injected (2×106 cells/mouse) into the axillary regions of mice. The mice are randomized to three groups: control, low-dose (10 mg/kg) or high-dose (20 mg/kg) of crenolanib (n = 6 per group) once the tumor volumes reach 70 mm3. 90% polyethylene glycol 300 and 10% 1-methyl-2-pyrrolidinone make up the delivery system for crenolanib therapy. For approximately two weeks, the tumor size and mouse body weight are measured every other day. The formula for calculating the tumor volume is (mm3)=(width×width×length)/2. Following therapy, carbon dioxide is used to kill the mice, and the tumors are removed and examined.
|
|
| References | ||
| Additional Infomation |
Crenolanib is a member of the class of benzimidazoles that is 1H-benzimidazole which is substituted by a 8-(4-aminopiperidin-1-yl)quinolin-2-yl group at position 1 and by a (3-methyloxetan-3-yl)methoxy group at position 5. It is an inhibitor of type III tyrosine kinases, PDGFRalpha/beta and FLT3 (IC50 of 11, 3.2, and 4 nM). Currently under clinical development for the treatment of acute myeloid leukemia. It has a role as an EC 2.7.10.1 (receptor protein-tyrosine kinase) inhibitor, an angiogenesis inhibitor, an antineoplastic agent and an apoptosis inducer. It is a member of benzimidazoles, an aromatic ether, a member of quinolines, a member of oxetanes, an aminopiperidine and a tertiary amino compound.
Crenolanib is under investigation for the treatment of Diffuse Intrinsic Pontine Glioma and Progressive or Refractory High-Grade Glioma. Crenolanib is an orally bioavailable benzimidazole targeting the platelet-derived growth factor receptor (PDGFR) subtypes alpha and beta and FMS-related tyrosine kinase 3 (Flt3), with potential antineoplastic activity. Upon oral administration, crenolanib binds to and inhibits both wild-type and mutated forms of PDGFR and Flt3, which may result in the inhibition of PDGFR- and Flt3-related signal transduction pathways. This results in inhibition of tumor angiogenesis and tumor cell proliferation in PDGFR and/or Flt3 overexpressing tumor cells. PDGFR and Flt3, class III receptor tyrosine kinases, are upregulated or mutated in many tumor cell types. |
| Molecular Formula |
C26H29N5O2
|
|---|---|
| Molecular Weight |
443.54
|
| Exact Mass |
443.232
|
| Elemental Analysis |
C, 70.41; H, 6.59; N, 15.79; O, 7.21
|
| CAS # |
670220-88-9
|
| Related CAS # |
670220-93-6 (besylate);670220-88-9;
|
| PubChem CID |
10366136
|
| Appearance |
Off-white to light green solid powder
|
| Density |
1.4±0.1 g/cm3
|
| Boiling Point |
676.6±65.0 °C at 760 mmHg
|
| Flash Point |
363.0±34.3 °C
|
| Vapour Pressure |
0.0±2.1 mmHg at 25°C
|
| Index of Refraction |
1.704
|
| LogP |
2.99
|
| Hydrogen Bond Donor Count |
1
|
| Hydrogen Bond Acceptor Count |
6
|
| Rotatable Bond Count |
5
|
| Heavy Atom Count |
33
|
| Complexity |
667
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
O1C([H])([H])C(C([H])([H])[H])(C([H])([H])OC2C([H])=C([H])C3=C(C=2[H])N=C([H])N3C2C([H])=C([H])C3C([H])=C([H])C([H])=C(C=3N=2)N2C([H])([H])C([H])([H])C([H])(C([H])([H])C2([H])[H])N([H])[H])C1([H])[H]
|
| InChi Key |
DYNHJHQFHQTFTP-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C26H29N5O2/c1-26(14-32-15-26)16-33-20-6-7-22-21(13-20)28-17-31(22)24-8-5-18-3-2-4-23(25(18)29-24)30-11-9-19(27)10-12-30/h2-8,13,17,19H,9-12,14-16,27H2,1H3
|
| Chemical Name |
1-[2-[5-[(3-methyloxetan-3-yl)methoxy]benzimidazol-1-yl]quinolin-8-yl]piperidin-4-amine
|
| Synonyms |
RO 002; ARO 002, CP-868596; ARO-002; CP 868596; CP868596; ARO002; RO-002; RO002
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 3 mg/mL (6.76 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 30.0 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 2: ≥ 1.43 mg/mL (3.22 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 14.3 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 1.43 mg/mL (3.22 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 4: 30% PEG400+0.5% Tween80+5% propylene glycol: 30mg/mL |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2546 mL | 11.2729 mL | 22.5459 mL | |
| 5 mM | 0.4509 mL | 2.2546 mL | 4.5092 mL | |
| 10 mM | 0.2255 mL | 1.1273 mL | 2.2546 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03258931 | Recruiting | Drug: Crenolanib Drug: Midostaurin |
Newly Diagnosed FLT3 Mutated AML |
Arog Pharmaceuticals, Inc. | August 15, 2018 | Phase 3 |
| NCT03250338 | Recruiting | Drug: Crenolanib Drug: Cytarabine |
Relapsed/Refractory Acute Myeloid Leukemia With FLT3 Activating Mutations |
Arog Pharmaceuticals, Inc. | June 5, 2018 | Phase 3 |
| NCT01393912 | Completed | Drug: Crenolanib | Progressive or Refractory High-Grade Glioma Diffuse Intrinsic Pontine Glioma |
St. Jude Children's Research Hospital |
July 2011 | Phase 1 |
| NCT02626364 | Completed | Drug: crenolanib | Recurrent/Refractory Glioblastoma | Arog Pharmaceuticals, Inc. | April 2016 | Phase 2 |
| NCT01243346 | Completed | Drug: Crenolanib besylate (CP-868,596-26) Dose: 140mg BID |
D842-related Mutant GIST | Arog Pharmaceuticals, Inc. | April 2011 | Phase 2 |
|
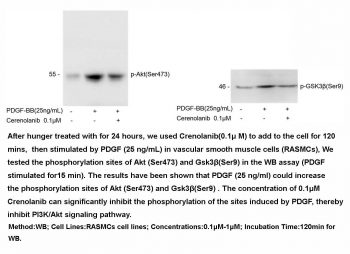 |
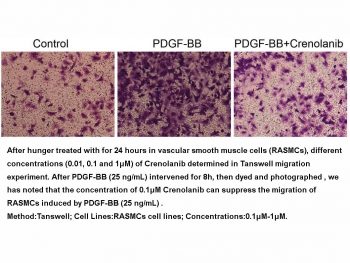 |