
| Size | Price | Stock | Qty |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
Purity: ≥98%
Clemastine Fumarate (HS-592; HS 592 fumarate; Meclastine), the fumarate salt of clemastine, is a first-generation H1 histamine antagonist with anticholinergic and sedative adverse effects. It has an IC50 of 3 nM for H1 histamine receptor inhibition. Clemastine is used as the hydrogen fumarate in hay fever, rhinitis, allergic skin conditions, and pruritus. It also relieves sneezing, runny nose, and red, itchy, and tearing eyes.
| Targets |
mTOR; Histamine H1 receptor ( IC50 = 3 nM )
- Histamine H₁ receptor (antagonist activity, no IC₅₀/Ki provided)[1,3,12] - Human Ether-à-go-go-Related Gene (hERG) K⁺ channel (IC₅₀ = 12 nM for blocking IHERG current)[7] - P2X₇ receptor (allosteric sensitizer, enhances ATP-induced cation entry)[8] |
|---|---|
| ln Vitro |
In vitro activity: Clemastine Fumarate inhibits the increase in [Ca2+]i caused by histamine in HL-60 cells, with an IC50 of 3 nM, whereas the IC50 values of chlorpheniramine and diphenhydramine are 20 nM and 100 nM, respectively.[1]
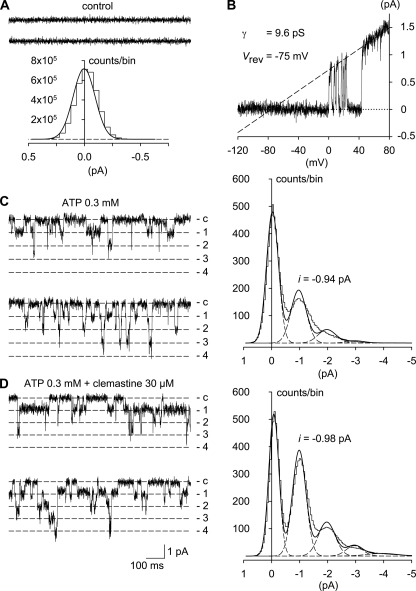
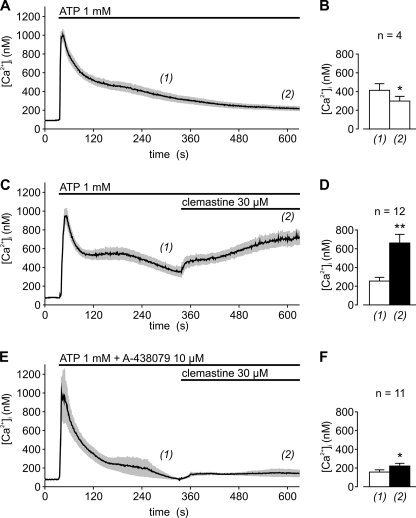
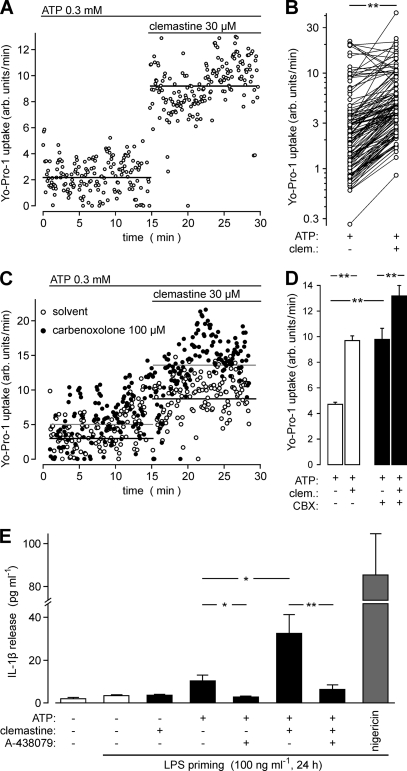
Clemastine fumarate dramatically suppresses lymphocyte NK and ADCC responses against human erythroleukemia cell line K562 and human B-lymphoblast cell line SB, respectively, at concentrations of ≥25 μM.[2] Clemastine Fumarate inhibits the contraction of the guinea pig ileum caused by histamine with an IC50 of 231 nM.[3] In HEK 293 cells that consistently express HERG channels, clemastine fumarate potently inhibits the HERG K+ channel in a concentration-dependent manner with an IC50 of 12 nM. This effect can be mitigated by the Y652A or F656A mutation of HERG.[4] Clemastine Fumarate augments the release of IL-1β from LPS-induced human macrophages and considerably amplifies the ATP-induced rise in [Ca2+]i in HEKhP2X7 cells, without depending on histamine receptor blockage but rather on sensitizing P2X7 receptor in a concentration-dependent manner with an EC50 of 10 μM.[5] - Antihistamine activity: Clemastine competitively blocks H₁ receptors on effector cells (e.g., vascular endothelium, airway smooth muscle), reducing histamine-mediated vasodilation and bronchoconstriction. This effect was confirmed in isolated guinea pig tracheal strips and rat mesenteric artery assays[3] - hERG channel inhibition: In HEK293 cells expressing hERG channels, clemastine (12 nM) significantly reduced peak IHERG current by ~50%, with voltage-dependent binding to the channel pore cavity. Mutations Y652A/F656A in the S6 helix attenuated this effect[7] - P2X₇ receptor modulation: In HEK293 cells stably expressing P2X₇ receptors, clemastine (1-10 μM) enhanced ATP-induced Ca²⁺ influx, accelerated pore dilation (Yo-Pro-1 uptake), and increased fractional permeability to NMDG⁺. Similar effects were observed in human monocyte-derived macrophages and murine bone marrow-derived macrophages[8] - Autophagy promotion: In LPS-stimulated H9c2 cardiomyocytes, clemastine (10-50 μM) increased LC3-II/LC3-I ratio, Beclin-1 expression, and autophagosome formation, which was abolished by the autophagy inhibitor 3-methyladenine. |
| ln Vivo |
Clemastine Fumarate treatment significantly lowers the innate immune responses of mice to Listeria monocytogenes by interfering with the production of proinflammatory cytokines like TNF-α and IL-6 by extracellular signal-regulated kinase (ERK). This effect is surprising because it is not dependent on blocking the histamine H1 receptor. The result is a significant increase in mortality.[7] Rats that are given Clemastine Fumarate (5–20 mg/kg) show a dose-dependent, significant inhibition of both zymosan paw oedema and croton oil ear oedema simultaneously. At 20 mg/kg, the inhibition is 53.6% and 46.8%, respectively, with ID50 values of 18.0 mg/kg and 20.5 mg/kg, respectively. [6]
Allergic rhinitis model: Oral clemastine (1 mg) administered 4-6 hours before allergen challenge significantly reduced sneezing frequency (p < 0.01) and rhinorrhea severity in 20 allergic subjects. The effect was dose-dependent and lasted ≥12 hours[6] - Sepsis-induced myocardial injury: In CLP-induced septic rats, intraperitoneal clemastine (30-50 mg/kg) improved 7-day survival rate (from 30% to 60%), reduced serum cTnI levels, preserved left ventricular ejection fraction, and attenuated mitochondrial fragmentation. Similar protective effects were observed in LPS-stimulated H9c2 cells - Optic neuritis model: Oral clemastine (1 mg twice daily for 90 days) in 16 patients with acute optic neuritis preserved retinal nerve fiber layer (RNFL) thickness in temporal/superotemporal quadrants and enhanced P100 wave amplitude recovery in visual evoked potentials compared to placebo. |
| Enzyme Assay |
In a buffer containing 138 mM NaCl, 6 mM KC1, 1 mM MgSO4, 1 mM Na2HPO4, 5 mM NaHCO33, 5.5 mM glucose, and 20 mM HEPES-NaOH, pH 7.4, HL-60 cells are suspended at a density of 1×107 cells/mL. The buffer is further enhanced with 0.1% (w/v) bovine serum albumin. Following a 10-minute incubation period at 37 °C, 4 μM of the dye fura-2/AM is added to the cells. After being diluted with the previously mentioned buffer to a concentration of 5×106 cells/mL, the cells are incubated at 37 °C for 45 minutes. Following this, cells are diluted using the previously mentioned buffer until they reach a final concentration of 0.5 × 106 cells/mL, and they are centrifuged at 250 g for 10 minutes at ambient temperature. In the previously mentioned buffer, cells are suspended at 1.0 × 106 cells/mL and stored at 20 °C until measurement. After loading with fura-2/AM, HL-60 cells are suspended in 2 mL of the previously mentioned buffer for a maximum of 4 hours using acryl fluorescence cuvettes. Prior to the addition of histamine (100 μM), HL-60 cells are incubated for 3 minutes at 37 °C with 1 mM Ca2+ and different concentrations of clemastine fumarate. With the cells constantly stirred at 1×103 rpm and at 37 °C, fluorescence is measured with a Ratio II spectrofluorometer. For one minute, the basal fluorescence, or basal [Ca2+]i, is measured. To find the increase in [Ca2+]i, the corresponding peak [Ca2+]i values are subtracted from the basal [Ca2+]i values. The wavelengths of excitation and emission are 500 nm and 340 nm, respectively. The competitive curve is used to calculate the IC50 value.
- hERG channel electrophysiology: Whole-cell patch-clamp recordings were performed on HEK293 cells expressing hERG channels. Cells were perfused with clemastine (1-100 nM) in Tyrode's solution at 37°C. IHERG tail currents were measured at -40 mV after depolarization to +20 mV. IC₅₀ was calculated using nonlinear regression[7] - P2X₇ receptor calcium flux assay: HEK293-P2X₇ cells were loaded with Fluo-4 AM and treated with clemastine (1-10 μM) followed by ATP (100 μM). Calcium transients were recorded using fluorescence microscopy. Yo-Pro-1 uptake was quantified by flow cytometry to assess pore formation[8] |
| Cell Assay |
- H₁ receptor antagonism assay: Guinea pig tracheal ring segments were incubated with clemastine (0.1-10 μM) and challenged with histamine (1 μM). Isometric tension changes were recorded to determine antagonist potency[3]
- Autophagy detection: H9c2 cardiomyocytes were treated with clemastine (10-50 μM) and LPS (1 μg/mL). LC3-II/LC3-I ratio and Beclin-1 expression were analyzed by Western blot. Autophagosomes were visualized by transmission electron microscopy. |
| Animal Protocol |
Male Wistar rats with paw oedema induced by subplantar injection of zymosan and ear oedema induced by croton oil
5-20 mg/kg Intraperitoneally - Sepsis model: Male Sprague-Dawley rats (250-300 g) underwent cecal ligation and puncture (CLP). Clemastine (10-50 mg/kg) was dissolved in 0.9% saline and administered intraperitoneally 30 minutes post-CLP. Survival was monitored for 7 days, and cardiac function was assessed by echocardiography on day 3 - Allergic rhinitis model: Human subjects received oral clemastine (1 mg) or placebo in a double-blind crossover design. Nasal allergen challenges were performed 1, 4, and 6 hours post-dose. Sneeze counts and nasal secretion weights were recorded[6] |
| ADME/Pharmacokinetics |
Absorption, Distribution and Excretion
Rapidly absorbed from the gastrointestinal tract. Urinary excretion is the major mode of elimination. Metabolism / Metabolites Antihistamines appear to be metabolized in the liver chiefly via mono- and didemethylation and glucuronide conjugation. - Absorption: Rapid oral absorption with ~40% bioavailability. Peak plasma concentration (Cmax) of 1-2 ng/mL occurs within 2-4 hours - Distribution: Extensive tissue distribution (volume of distribution ~800 L), crosses blood-brain barrier. Plasma protein binding ~95% - Metabolism: Extensively metabolized in liver via O-dealkylation, oxidation, and glucuronidation. Major metabolites include desmethylclemastine and hydroxylated derivatives - Excretion: ~42% excreted in urine (primarily as metabolites), 27% in feces. Terminal half-life ~21 hours. |
| Toxicity/Toxicokinetics |
Hepatotoxicity
Despite widespread use, the first generation antihistamines such as clemastine have rarely been linked to liver test abnormalities or to clinically apparent liver injury. The reason for their safety may relate to low daily dose and limited duration of use. Likelihood score: E (unlikely to be a cause of clinically apparent liver injury). References on the safety and potential hepatotoxicity of antihistamines are given together after the Overview section on Antihistamines. Drug Class: Antihistamines Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Small occasional doses of clemastine are acceptable during breastfeeding. Larger doses or more prolonged use may cause drowsiness and other effects in the infant or decrease the milk supply, particularly in combination with a sympathomimetic such as pseudoephedrine or before lactation is well established. Single bedtime doses after the last feeding of the day may be adequate for many women and will minimize any effects of the drug. The nonsedating antihistamines are preferred alternatives. ◉ Effects in Breastfed Infants A 10-week-old breastfed infant whose mother was taking clemastine, phenytoin and carbamazepine was drowsy, refused to feed, was irritable, and had high-pitched crying. These side effects were possibly caused by clemastine in breastmilk, but the other drugs could also have contributed. In one telephone follow-up study, mothers reported irritability and colicky symptoms 10% of infants exposed to various antihistamines and drowsiness was reported in 1.6% of infants. None of the reactions required medical attention. ◉ Effects on Lactation and Breastmilk Antihistamines in relatively high doses given by injection can decrease basal serum prolactin in nonlactating women and in early postpartum women. However, suckling-induced prolactin secretion is not affected by antihistamine pretreatment of postpartum mothers. Whether lower oral doses of antihistamines have the same effect on serum prolactin or whether the effects on prolactin have any consequences on breastfeeding success have not been studied. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Non-Human Toxicity Values LD50 Mouse oral 730 mg/kg LD50 Mouse iv 43 mg/kg LD50 Rat oral 3550 mg/kg LD50 Rat iv 82 mg/kg. - Acute toxicity: LD₅₀ in mice >100 mg/kg (oral). Common adverse effects include sedation, dry mouth, blurred vision, and urinary retention due to anticholinergic activity - Cardiotoxicity: At supra-therapeutic concentrations (≥1 μM), clemastine prolongs QT interval in isolated feline hearts, but no clinical QT prolongation reported at therapeutic doses (1-6 mg/day)[7] - Drug interactions: Potentiates CNS depression with alcohol, opioids, or benzodiazepines. Contraindicated with MAO inhibitors due to risk of hypertensive crisis. |
| References |
[1]. Mol Pharmacol . 1992 Aug;42(2):227-34. [2]. Cell Immunol . 1983 Oct 1;81(1):45-60. [3]. J Pharmacol Exp Ther . 1997 Jan;280(1):114-21. [4].J Mol Cell Cardiol . 2006 Jan;40(1):107-18. [5]. J Biol Chem . 2011 Apr 1;286(13):11067-81. [6]. J Pharmacol Exp Ther . 1997 Jan;280(1):114-21. |
| Additional Infomation |
Clemastine is 2-[(2R)-1-Methylpyrrolidin-2-yl]ethanol in which the hydrogen of the hydroxy group is substituted by a 1-(4-chlorophenyl)-1-phenylethyl group (R configuration). An antihistamine with antimuscarinic and moderate sedative properties, it is used as its fumarate salt for the symptomatic relief of allergic conditions such as rhinitis, urticaria, conjunctivitis and in pruritic (severe itching) skin conditions. It has a role as a H1-receptor antagonist, an anti-allergic agent, a muscarinic antagonist and an antipruritic drug. It is a N-alkylpyrrolidine and a member of monochlorobenzenes.
An ethanolamine-derivative, first generation histamine H1 antagonist used in hay fever, rhinitis, allergic skin conditions, and pruritus. It causes drowsiness. Clemastine is a first generation antihistamine that is used for symptoms of allergic rhinitis and the common cold. Clemastine has not been linked to instances of clinically apparent acute liver injury. Clemastine is a synthetic ethanolamine, with anticholinergic, sedative, and histamine H1 antagonistic activities. Upon administration, clemastine blocks the H1 histamine receptor and prevents the symptoms that are caused by histamine activity on capillaries, bronchial and gastrointestinal smooth muscles, including vasodilation, increased capillary permeability, bronchoconstriction, and spasmodic contraction of gastrointestinal smooth muscles. Clemastine also prevents histamine-induced pain and itching of mucous membranes. A histamine H1 antagonist used as the hydrogen fumarate in hay fever, rhinitis, allergic skin conditions, and pruritus. It causes drowsiness. See also: Clemastine Fumarate (has salt form). Drug Indication For the relief of symptoms associated with allergic rhinitis such as sneezing, rhinorrhea, pruritus and acrimation. Also for the management of mild, uncomplicated allergic skin manifestations of urticaria and angioedema. Used as self-medication for temporary relief of symptoms associated with the common cold. Mechanism of Action Clemastine is a selective histamine H1 antagonist and binds to the histamine H1 receptor. This blocks the action of endogenous histamine, which subsequently leads to temporary relief of the negative symptoms brought on by histamine. Therapeutic Uses For the symptomatic treatment of allergic rhinitis in adults and children 12 years of age and older ... For the symptomatic treatment of mild allergic urticaria and angioedema in adults and children 12 years of age and older ... This multicenter, double blind, randomized parallel group study compared 3 wk treatment with either loratadine (Clarityn) 10 mg once daily, or clemastine (Tavegyl) 1 mg twice daily, and placebo in outpatients with active perennial allergic rhinitis. 155 patients were evaluated for efficacy and safety. Grading of four nasal and three non-nasal symptoms, rhinoscopy signs, and therapeutic response was performed on treatment days 6, 13, and 20. Patients recorded daily symptoms and possible adverse experiences in a diary, also indicating when symptoms of active rhinitis were relieved. Loratadine and clemastine were statistically significantly superior to placebo throughout the study (p < 0.05), based on assessment of patients' nasal and eye symptoms, patients' diary scores, rhinoscopy signs of symptoms, and onset of relief. The loratadine group showed a statistically significantly (p < 0.05) faster onset of relief of symptoms compared with the group treated with clemastine. Concerning nasal stuffiness, loratadine was significantly (p < 0.05) superior to clemastine after 1 week's treatment. Reports of adverse reactions showed that significantly (p < 0.05) more patients complained of sedation in the clemastine than in the loratadine group. Regarding other adverse experiences and laboratory tests, the three treatment groups were statistically comparable (p < 0.05). The study showed that compared with placebo both loratadine and clemastine were effective in relieving nasal and eye symptoms in patients with perennial allergic rhinitis. Loratadine was safe and well tolerated and was significantly less sedative than clemastine; loratadine may therefore possess an advantage in clinical use in the treatment of perennial allergic rhinitis. The effects of cromolyn sodium (sodium cromoglycate), clemastine and ketotifen administered to the nasal mucosa of seasonal and perennial allergic rhinitis patients 30 min before provocation with histamine and allergen were compared in a randomized, double blind, placebo controlled study. Clemastine and cromolyn sodium, but not ketotifen, significantly inhibited the nasal response to increasing concentrations of histamine. None of the drugs administered in the concentrations used significantly inhibited the nasal response to allergen. Drug Warnings Drugs contraindicated during lactation include ... clemastine. There are no adequate and controlled studies to date using clemastine fumarate alone or in fixed combination with phenylpropanolamine in pregnant women, and the drug should be used during pregnancy only when clearly needed. Because of the potential for serious adverse reactions to clemastine in nursing infants, a decision should be made whether to discontinue nursing or the drug, taking into account the importance of the drug to the woman. Drugs that have been associated with Significant Effects on some Nursing Infants and should be given to Nursing Mothers with Caution: Clemastine: Drowsiness, irritability, refusal to feed, high-pitched cry, neck stiffness (1 case). /from Table 5/ |
| Molecular Formula |
C25H30CLNO5
|
|
|---|---|---|
| Molecular Weight |
459.96
|
|
| Exact Mass |
459.181
|
|
| Elemental Analysis |
C, 65.28; H, 6.57; Cl, 7.71; N, 3.05; O, 17.39
|
|
| CAS # |
14976-57-9
|
|
| Related CAS # |
Clemastine; 15686-51-8; Clemastine-d5 fumarate
|
|
| PubChem CID |
26987
|
|
| Appearance |
White to light yellow crystalline powder
|
|
| Density |
1.097 g/cm3
|
|
| Boiling Point |
116 °C / 24mmHg
|
|
| Melting Point |
61 °C
|
|
| Flash Point |
211ºC
|
|
| Vapour Pressure |
1.94E-07mmHg at 25°C
|
|
| Index of Refraction |
1.553
|
|
| LogP |
4.754
|
|
| Hydrogen Bond Donor Count |
0
|
|
| Hydrogen Bond Acceptor Count |
2
|
|
| Rotatable Bond Count |
6
|
|
| Heavy Atom Count |
24
|
|
| Complexity |
376
|
|
| Defined Atom Stereocenter Count |
2
|
|
| SMILES |
ClC1C([H])=C([H])C(=C([H])C=1[H])[C@@](C([H])([H])[H])(C1C([H])=C([H])C([H])=C([H])C=1[H])OC([H])([H])C([H])([H])[C@@]1([H])C([H])([H])C([H])([H])C([H])([H])N1C([H])([H])[H].O([H])C(/C(/[H])=C(\[H])/C(=O)O[H])=O
|
|
| InChi Key |
PMGQWSIVQFOFOQ-YKVZVUFRSA-N
|
|
| InChi Code |
InChI=1S/C21H26ClNO.C4H4O4/c1-21(17-7-4-3-5-8-17,18-10-12-19(22)13-11-18)24-16-14-20-9-6-15-23(20)2;5-3(6)1-2-4(7)8/h3-5,7-8,10-13,20H,6,9,14-16H2,1-2H3;1-2H,(H,5,6)(H,7,8)/b;2-1+/t20-,21-;/m1./s1
|
|
| Chemical Name |
(E)-but-2-enedioic acid;(2R)-2-[2-[(1R)-1-(4-chlorophenyl)-1-phenylethoxy]ethyl]-1-methylpyrrolidine
|
|
| Synonyms |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month Note: Please store this product in a sealed and protected environment, avoid exposure to moisture. |
|
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 1.43 mg/mL (3.11 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 14.3 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 1.43 mg/mL (3.11 mM) (saturation unknown) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 14.3 mg/mL clear DMSO stock solution to 900 μL of 20% SBE-β-CD physiological saline solution and mix evenly. Preparation of 20% SBE-β-CD in Saline (4°C,1 week): Dissolve 2 g SBE-β-CD in 10 mL saline to obtain a clear solution. View More
Solubility in Formulation 3: ≥ 1.43 mg/mL (3.11 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. Solubility in Formulation 4: 30% propylene glycol, 5% Tween 80, 65% D5W: 5mg/mL Solubility in Formulation 5: 1.43 mg/mL (3.11 mM) in PBS (add these co-solvents sequentially from left to right, and one by one), clear solution; with ultrasonication (<60°C). |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1741 mL | 10.8705 mL | 21.7410 mL | |
| 5 mM | 0.4348 mL | 2.1741 mL | 4.3482 mL | |
| 10 mM | 0.2174 mL | 1.0871 mL | 2.1741 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT06039137 | Active Recruiting |
Drug: Cetirizine | Solid Tumor | Erasmus Medical Center | June 1, 2022 | N/A |
| NCT03109288 | Recruiting | Drug: Pioglitazone Drug: clemastine fumarate Drug: Dantrolene Drug: Pirfenidone |
Multiple Sclerosis | National Institute of Allergy and Infectious Diseases (NIAID) |
August 11, 2017 | Phase 1 Phase 2 |
| NCT02521311 | Recruiting | Drug: Clemastine Drug: Placebo |
Optic Neuritis | University of California, San Francisco |
February 28, 2017 | Phase 2 |
| NCT05359653 | Recruiting | Drug: Clemastine Fumarate Drug: Placebo |
Multiple Sclerosis (MS) Multiple Sclerosis Relapse Multiple Sclerosis Benign |
University of California, San Francisco |
August 1, 2023 | Phase 1 Phase 2 |
| NCT06065670 | Not yet recruiting | Drug: Clemastine Fumarate Drug: Placebo |
Demyelinating Diseases Multiple Sclerosis Brain Lesion |
University of California, San Francisco |
November 1, 2023 | Phase 1 Phase 2 |
 |
|---|
 |
 |