
| Size | Price | Stock | Qty |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
Purity: ≥98%
CH5132799 is a novel and potent class I PI3K (phosphoinositide 3-kinase) inhibitor with potential anticancer activity. With an IC50 of 14 nM, it inhibits class I PI3Ks, specifically PI3K, and is less potent against PI3K while being sensitive in PIK3CA mutant cell lines. With IC50 values for PI3K, PI3K, PI3K, and PI3K of 0.014 M, 0.12 M, 0.5 M, and 0.036 M, respectively, CH5132799 exhibits inhibitory effects against class I PI3K.
| Targets |
PI3Kα (IC50 = 180 nM); PI3Kα-H1047R (IC50 = 5.6 nM); PI3Kα-E545K (IC50 = 6.7 nM); PI3Kα-E542K (IC50 = 6.7 nM); PI3Kγ (IC50 = 36 nM); PI3Kβ (IC50 = 120 nM); PI3Kδ (IC50 = 500 nM); PI3KC2β (IC50 = 5.3 μM); mTOR (IC50 = 1.6 μM)
|
|---|---|
| ln Vitro |
CH5132799 selectively inhibits class I PI3Ks, PI3Kα (IC50 = 0.014 μM), PI3Kβ (IC50 = 0.12 μM), PI3Kδ (IC50 = 0.50 μM), PI3Kγ (IC50 = 0.036 μM), but shows less inhibition of class II PI3Ks, class III PI3k and mTOR and also no inhibitory activity (IC50 > 10 μM) against 26 protein kinases. In comparison to wild-type PI3Kα, CH5132799 has stronger inhibitory effects against PI3Kα with the oncogenic mutations E542K (IC50 = 6.7 nM), E545K (IC50 = 6.7 nM), and H1047R (IC50 = 5.6 nM). CH5132799 treated breast cnacer KPL-4 cells, which harbor the PIK3CA mutation, phosphorylation of Akt and its direct substrates, PRAS40 and FoxO1/3a and phosphorylation of downstream factors, including S6K, S6 and 4E-BP1, are effectively suppressed. Cancer cell lines with PIK3CA mutations are significantly more sensitive to CH5132799 [1].The BAC-TO-BAC Baculovirus Expression System is used in conjunction with PI3K mutants that have been Glutathione S-transferase-tagged and His-tagged p85. Using the Adapta Universal Kinase Assay Kit, CH5132799's inhibitory effects on PI3K (p110/p85), PI3K (p110/p85), PI3K (p110), PI3KC2, PI3KC2, Vps34, and PI3K mutants are assessed. An EnVision HTS microplate reader is used to measure time-resolved fluorescence. Utilizing XLfit, IC50 values are determined.
|
| ln Vivo |
CH5132799 shows potent in vivo antitumor activity in several different xenograft models with PIK3CA mutations. CH5132799 overcomes mTORC1 inhibition-mediated Akt activation and regrowth of xenograft tumor by long-term everolimus administration.[1]
|
| Enzyme Assay |
The E542K, E545K, and H1047R mutants of PI3Kα are prepared with an overlapped extension-polymerase chain reaction. The cell lines are added to the wells of 96-well plates containing 0.076 to 10,000 nM CH5132799 and incubated at 37 °C. After 4 days of incubation, Cell Counting Kit-8 solution is added and, after incubation for several more hours, absorbance at 450 nm is measured with Microplate-Reader iMark. The antiproliferative activity is calculated by the formula (1- T/C) × 100 (%), in which T and C represent absorbance at 450 nm of the cells treated with CH5132799 (T) and that of untreated control cells (C). The IC50 values are calculated by using Microsoft Excel 2007.
|
| Cell Assay |
The cell lines are put into the 96-well plates with 0.076 to 10,000 nM CH5132799 and then incubated at 37 °C. Cell Counting Kit-8 solution is added after 4 days of incubation, and then Microplate-Reader iMark is used to measure absorbance at 450 nm. The formula (1- T/C) 100 (%) is used to determine the antiproliferative activity, where T and C stand for the absorbance at 450 nm of control cells that were left untreated and CH5132799-treated cells, respectively. Microsoft Excel 2007 is used to calculate the IC50 values.
|
| Animal Protocol |
Mice: The mice used are female BALB-nu/nu (CAnN.Cg-Foxn1/CrlCrlj nu/nu) mice. Subcutaneous injection of 4106 to 1.2107 cells, suspended in 100 to 200 L of serum-free culture medium, is performed in the right flank of the mice. Tumor volume (TV) is determined after measuring the size of the tumor twice per week with a gauge. Animals are divided into groups at random (n=4 or 5) once the tumors have reached a volume of roughly 200 to 300 mm3 and treatment has begun. Once daily oral administration of CH5132799, Everolimus, and Trastuzumab is combined with a weekly intravenous injection of Trastuzumab.
|
| References | |
| Additional Infomation |
CH5132799 has been used in trials studying the treatment of Solid Tumors.
Izorlisib is an orally bioavailable inhibitor of the class I phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) catalytic subunit alpha (PIK3CA), with potential antineoplastic activity. Upon administration, izorlisib selectively binds to and inhibits PIK3CA and its mutated forms in the PI3K/Akt (protein kinase B)/mammalian target of rapamycin (mTOR) pathway. This results in both apoptosis and growth inhibition in PIK3CA-expressing tumor cells. By specifically targeting PIK3CA, izorlisib may be more efficacious and less toxic than pan-PI3K inhibitors. In addition, izorlisib also targets mutated forms of PI3K gamma (PI3Kg). It may also stimulate the immune system to restore CD8+ T-cell activation and cytotoxicity. Dysregulation of the PI3K/Akt/mTOR pathway is often found in solid tumors and results in the promotion of tumor cell growth, survival, and resistance to chemo- and radio-therapy. PIK3CA, one of the most frequently mutated oncogenes, encodes the p110-alpha catalytic subunit of the class I PI3K. In most solid tumors, the activation of the PI3K pathway is induced by mutations of PIK3CA. |
| Molecular Formula |
C15H19N7O3S
|
|---|---|
| Molecular Weight |
377.42
|
| Exact Mass |
377.127
|
| Elemental Analysis |
C, 47.73; H, 5.07; N, 25.98; O, 12.72; S, 8.50
|
| CAS # |
1007207-67-1
|
| Related CAS # |
1007207-67-1;2242053-82-1 (mesylate);
|
| PubChem CID |
49784945
|
| Appearance |
White to gray solid powder
|
| Density |
1.6±0.1 g/cm3
|
| Boiling Point |
751.1±70.0 °C at 760 mmHg
|
| Flash Point |
408.1±35.7 °C
|
| Vapour Pressure |
0.0±2.5 mmHg at 25°C
|
| Index of Refraction |
1.706
|
| LogP |
-0.87
|
| Hydrogen Bond Donor Count |
1
|
| Hydrogen Bond Acceptor Count |
10
|
| Rotatable Bond Count |
3
|
| Heavy Atom Count |
26
|
| Complexity |
587
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
S(C([H])([H])[H])(N1C2C(=C(C3=C([H])N=C(N([H])[H])N=C3[H])N=C(N=2)N2C([H])([H])C([H])([H])OC([H])([H])C2([H])[H])C([H])([H])C1([H])[H])(=O)=O
|
| InChi Key |
JEGHXKRHKHPBJD-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C15H19N7O3S/c1-26(23,24)22-3-2-11-12(10-8-17-14(16)18-9-10)19-15(20-13(11)22)21-4-6-25-7-5-21/h8-9H,2-7H2,1H3,(H2,16,17,18)
|
| Chemical Name |
5-(7-(methylsulfonyl)-2-morpholino-6,7-dihydro-5H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrimidin-2-amine
|
| Synonyms |
PA-799; PA799; PA-799; Izorlisib; CH5132799; CH-5132799; CH 5132799
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
|
|||
|---|---|---|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 0.46 mg/mL (1.22 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 4.6 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. Solubility in Formulation 2: 1% DMSO +30% polyethylene glycol+1% Tween 80 : 30mg/mL (Please use freshly prepared in vivo formulations for optimal results.) |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6496 mL | 13.2478 mL | 26.4957 mL | |
| 5 mM | 0.5299 mL | 2.6496 mL | 5.2991 mL | |
| 10 mM | 0.2650 mL | 1.3248 mL | 2.6496 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Status | Interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01222546 | Completed | Drug: CH5132799 | Solid Tumors | Chugai Pharma Europe Ltd. | August 2010 | Phase 1 |
Inhibition of PI3K pathway signaling in cells. KPL-4 cells were treated with the indicated concentrations of CH5132799 for 2 hours. Clin Cancer Res, 2011, 17(10), 3272-3281. |
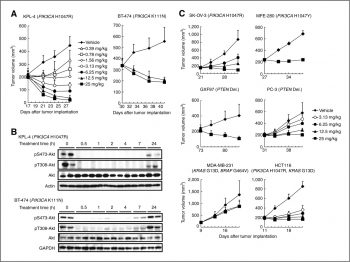 Antitumor activity in mouse xenograft models of cell lines harboring genetic alterations, including PIK3CA mutations |
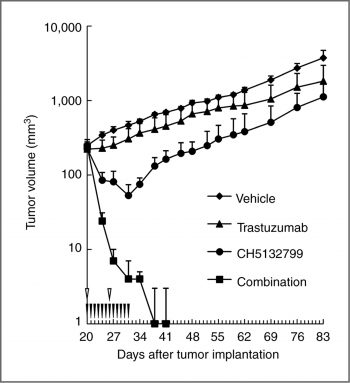 Antitumor activity in combination with trastuzumab in the trastuzumab-insensitive model. |