
| Size | Price | Stock | Qty |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
Purity: ≥98%
Dactolisib (formerly also known as NVP-BEZ235; BEZ235; BEZ-235) is a novel, potent and dual ATP-competitive inhibitor of PI3K (phosphatidylinositol 3-kinase) and mTOR for p110α/γ/δ/β and mTOR(p70S6K) anticancer activity. With IC50 values of 4 nM, 5 nM, 7 nM, 75 nM, and 6 nM in cell-free assays, it inhibits mTOR for p110/// and mTOR(p70S6K). The first PI3K inhibitor to enter clinical trials was dactolisib (introduced in 2006), which exhibits strong antitumor efficacy against nonfunctioning pituitary adenomas. The IC50 value for it in 3T3TopBP1-ER cells is 21 nM, and it inhibits ATR. BEZ235 has both in vitro and in vivo evidence of potential anti-tumor activity. Regardless of the PI3K pathway mutation status, it stopped the growth of several cancer cell lines. It exhibited antitumor activity in xenograft mouse models and blocked PI3K signaling. It was shown through a combination study that temozolomide's effectiveness is increased.
| Targets |
p110α (IC50 = 4 nM); p110α-H1047R (IC50 = 4.6 nM); p110α-E545K (IC50 = 5.7 nM); p110γ (IC50 = 5 nM); p110δ (IC50 = 7 nM); p110β (IC50 = 75 nM); mTOR (IC50 = 20.7 nM); mTORC1; mTORC2; Autophagy
|
|---|---|
| ln Vitro |
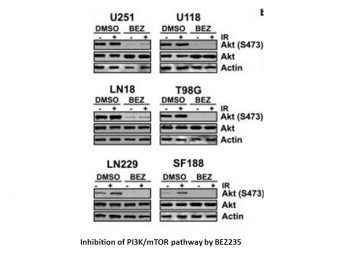
Dactolisib (BEZ235) potently inhibits PI3K in an ATP Competitive Manner. The phosphorylation levels of p70S6K, an mTOR activated kinase, were significantly lowered by the 250 nM dose of dactolisib (BEZ235). Due to the fact that the kinase domain of mTOR is highly similar to the one of class IA PI3K, Dactolisib (BEZ235) also causes a decrease in S235/S236P-RPS6 levels with an IC50 of 6.5 nM. A biochemical mTOR K-LISA assay (IC50, 20.7 nM) is used to demonstrate Dactolisib's (BEZ235) anti-mTOR activity[1].
The IC50s of Dactolisib (BEZ235) for the HCT116, DLD-1, and SW480 cell lines are 14.36.4, 9.01.5, and 12.01.6 nM, respectively[2]. NVP-BEZ235 Potently Inhibits PI3K in an ATP Competitive Manner. NVP-BEZ235 Specifically Blocks the PI3K Pathway in Cells. Effects of NVP-BEZ235 on Akt Downstream Effectors and mTOR. NVP-BEZ235 Possesses Strong Antiproliferative Activity. [1] In vitro NVP-BEZ235 treatment of human CRC cell lines decreases cellular proliferation but has no effect on apoptosis. In vitro NVP-BEZ235 treatment of human CRC cell lines results in sustained mTORC1 and mTORC2 inhibition, but transient PI3K blockade. The efficacy of in vitro NVP-BEZ235 treatment of human CRC cell lines does not depend on PIK3CA mutational status [2]. |
| ln Vivo |
Dactolisib (BEZ235) (45 mg/kg, p.o.) treatment induces colonic tumor regression in a GEM model for sporadic PIK3CA wild-type CRC[2]. Dactolisib (BEZ235) is given orally to MENX rats (n=2 per group) at a dose of 45 mg/kg, and the animals are sacrificed 1 or 6 hours later. When compared to rats treated with PEG, immunostains for P-AKT and P-S6 show a significant reduction of the two proteins, particularly P-S6, 6 hours after Dactolisib (BEZ235) administration. Pituitary adenomas in Dactolisib (BEZ235)-treated rats have a proteomic profile that is significantly different from tumors in placebo-treated rats six hours after treatment[3].
Dactolisib (BEZ235, NVP-BEZ 235) Is an Orally Available PI3K Inhibitor with Well-Tolerated Antitumor Activity [1] The pharmacokinetic properties of NVP-BEZ235 were originally evaluated in PC3M tumor-bearing nude mice. At a dose of 50 mg/kg, NVP-BEZ235 appeared rapidly in plasma with a Cmax of 1.68 μmol/L at 0.5 h and a C24h of 0.03 μmol/L. In the tumor tissue, the Cmax attained was 2.05 nmol/g at 1 h (Tmax), which decreased to 0.23 nmol/g after 24 h (Fig. 5A). Dactolisib (BEZ235, NVP-BEZ 235) is eliminated relatively quickly from the liver. In vivo analysis of the S473P-Akt levels in the tumor tissue revealed that maximum inhibition was obtained 1 h after dosing (corresponding to the tumor Cmax), and persistent inhibition was observed still 16 h after treatment, with almost complete recovery to basal levels obtained in two of four tumors at 24 h after dose (Fig. 5B). Pharmacokinetic simulation based on this study revealed that steady-state levels would be achieved between 3 and 5 days, when dosing either at 50 mg/kg given daily or at 25 mg/kg given twice daily. Differences between the dosage regimens would reside in the predicted tumor peak levels (2.6 versus 1.6 nmol/g, respectively) whereas through levels would remain almost comparable (0.53 versus 0.60 μmol/L, respectively). Taking into consideration this pharmacokinetic simulation, chronic treatment of PC3M tumor-bearing animals was done at 25 mg/kg p.o. NVP-BEZ235 twice daily. Using this schedule, a statistically significant inhibition of tumor growth was observed, with a final T/C value of 22% after 10 days of treatment (Fig. 5C). The treatment was well tolerated as concluded from the nonstatistically significant effect of NVP-BEZ235 on body weight gain (Fig. 5C) and by the fact that none of the animals died during the course of the study. The antitumor effect was well correlated with the inhibition of S473P-Akt in the tumor tissue 1 or 18 h after the last dose detected either by Western blotting of tumor extracts or by immunostaining of tumor sections (Fig. 5D). Compound concentrations at these time points were 1.32 nmol/g at 1 h and 0.51 nmol/g at 18 h, close to the predicted values for steady-state peak levels (see above). In vivo Dactolisib (BEZ235, NVP-BEZ 235) treatment of a GEM model for sporadic CRC results in sustained mTORC1 and mTORC2 inhibition, but transient PI3K blockade [2] To examine the effects of in vivo Dactolisib (BEZ235, NVP-BEZ 235) treatment on PI3K and mTOR signaling, western blot analysis was performed in colonic tumors that were harvested one hour after final drug dosing on days 5 and 28 of treatment. Western blot analysis for levels of p-AKTThr308, p-S6Ser240/244, and p-AKTSer473 was performed as surrogates for activation of the PI3K, mTORC1, and mTORC2 pathways, respectively. A sustained decrease in levels of p-AKTSer473 and p-S6Ser240/244 was observed in the tumors of mice treated with NVP-BEZ235, as compared to control diluent (Figure 4A and 4B). These findings were confirmed by tumor immunohistochemistry (Figure 4C). As with our in vitro studies, an initial decrease in levels of p-AKTThr308 was observed at five days after treatment, but normalized by 28 days (Figures 4A and 4B). Taken together, these results suggest that in vivo NVP-BEZ235 treatment results in sustained inhibition mTORC1 and mTORC2, but transient PI3K blockade. |
| Enzyme Assay |
PI3Kα, β, and δ proteins are made up of the iSH2 domain of p85 fused to the complete protein p110, with the exception of, which likewise lacks the final 20 amino acids. Full-length PI3K proteins with the first 144 amino acids deleted are generated. For easy purification, each construct is fused to a COOH-terminal His tag before being cloned into the pBlue-Bac4.5 or pVL1393 plasmids, depending on the isoform being studied. Then, using techniques suggested by the vendor for producing the appropriate recombinant baculoviruses and proteins, the various vectors are cotransfected with BaculoGold WT genomic DNA. A Kinase-Glo assay is used to assess BEZ235's ability to inhibit PI3K. The 384-well black plate is used for the kinase reaction. The PI3K proteins (10, 25, 10, and 150 nM of p110, p110, p110, and p110, respectively) are then added to each well after it has been loaded with 50 L of test items (in 90% DMSO) and 5 L of reaction buffer containing 10 g/mL PI substrate (l-phosphatidylinositol; Avanti Polar Lipids). 5 L of 1 M ATP prepared in the reaction buffer is added to begin the reaction, which is then incubated for 60 (for p110, p110, and p110) or 120 (for p110) minutes. The addition of 10 L of Kinase-Glo buffer ends the reaction. The plates are then examined for luminescence using a Synergy 2 reader. The 384-well black plate is used for the kinase reaction. The PI3K proteins (10, 25, 10, and 150 nM of p110α, p110β, p110δ, and p110γ, respectively) are then added to each well after it has been loaded with 50 μL of test items (in 90% DMSO) and 5 μL of reaction buffer containing 10 μg/mL PI substrate (l-phosphatidylinositol; Avanti Polar Lipids). 5 μL of 1 μM ATP prepared in the reaction buffer is added to begin the reaction, which is then incubated for 60 (for p110α, p110β, and p110δ) or 120 min (for p110γ) minutes. The addition of 10 μL of Kinase-Glo buffer ends the reaction. The plates are then examined for luminescence using a Synergy 2 reader.
|
| Cell Assay |
The human CRC cell lines, HCT116 (PIK3CA mutant; kinase domain at H1047R), DLD-1 (PIK3CA mutant; helical domain at E545K), and SW480 (PIK3CA wild-type) and isogenic DLD-1 PIK3CA mutant as well as wild-type cells are maintained in DMEM with 10% FBS and 1 × Penicillin/Streptomycin. To account for different growth kinetics, cells are plated at various initial densities (HCT116: 3 103 cells/well, DLD-1: 5.5 103 cells/well, SW480: 4.5 103 cells/well, DLD-1 PIK3CA mutant: 7 103 cells/well, and DLD-1 PIK3CA wild-type: 9 103 cells/well). BEZ235 is added to the cells at increasing concentrations after 16 hours, and the growth medium containing the drug is changed every 24 hours. According to the manufacturer's recommendations, cell viability is measured 48 hours after the start of drug treatment and 16 hours after the initial plating using the colorimetric MTS assay CellTiter 96® AQueous One Solution Cell Proliferation Assay. Cell viability following drug treatment is compared to untreated cells that were grown for 48 hours. Cells are plated with either no BEZ235 or the maximum inhibitory dose (500 nM) for 2, 6, 24, or 48 hours before being subjected to a western blot analysis.
|
| Animal Protocol |
Mice: An oral gavage of 45 mg/kg of BEZ235 in 10% 1-methyl-2-pyrrolidone/90% PEG 300 is administered daily for 28 days to tumor-bearing Apc CKO mice or a control vehicle alone (n = 8) for treatment. Based on evidence from the literature, the recommended dose of BEZ235 is 40–50 mg/kg body weight, which has been shown to be safe and effective in treating murine tumor models. Tumor-bearing mice are sacrificed one hour after the last dose of the drug, in accordance with pharmacokinetic studies showing that the tissue concentration of NVP-BEZ235 reaches its peak one hour after administration. Utilizing calipers to measure the width, length, and height of the colonic tumor, tumors are harvested for immunohistochemistry and western blot analysis.
Rats: MENX-affected rats used. In MENX rats, BEZ235 is tested at doses of 20, 30, and 45 mg/kg. The dose of 20 mg/kg is used for further studies because the two higher doses result in a weight loss of greater than 10% after 10 days of treatment. For MRI studies, BEZ235 (20 mg/kg) or a placebo (PEG) are given orally once daily to MENX-affected rats aged 7 to 8 months who have sizeable adenomas but are otherwise healthy. Establishment of Xenograft Tumors, Efficacy Studies, Compound Preparation, and Analytics [1] Establishment of tumors, group randomization, tumor, and body weight recording during efficacy studies were described elsewhere. Antitumor activity is expressed as %T/C (mean increase of tumor volumes of treated animals divided by the mean increase of tumor volumes of control animals multiplied by 100) and/or as tumor regression (%Reg) calculated as [(mean tumor volume at the start of treatment - mean tumor volume) / (mean tumor volume at the start of treatment)] × 100. Data are presented as mean ± 1 SE. Comparisons between groups and vehicle control group were done using either one-way ANOVA or ANOVA on ranks followed by Dunnett's tests when data were respectively either normally distributed or not. For all tests, the level of significance was set at P < 0.05. Calculations were done using SigmaStat version 2.03. Dactolisib (BEZ235, NVP-BEZ 235) (free base) was formulated in NMP/polyethylene glycol 300 (10/90, v/v). Solutions (5 mg/mL) were prepared fresh each day of dosing as follows: the powder was dissolved in NMP on sonication, and the remaining volume of polyethylene glycol 300 was added. The application volume was 10 mL/kg. For analytics, frozen tissues were minced and then homogenized in an equal volume of ice-cold PBS using a Polytron homogenizer (IKA). After acetonitrile precipitation and centrifugation, supernatants were analyzed by reverse-phase high-performance liquid chromatography/UV on a Merck-Hitachi/LaChrom equipment including a Nucleosil 100-5 C18 column. Samples were then eluted with a linear gradient of 10% to 90% (v/v) acetonitrile in water containing 0.05% (v/v) trifluoroacetic acid over a period of 20 min at a flow rate of 1 mL/min. The compounds were detected by UV absorbance at 340 nm, and concentrations were determined by the external standard method using peak heights. In vivo treatment of a GEM model for sporadic CRC [2] Apc CKO mice were treated with Adeno-Cre and followed by optical colonoscopy, as previously described [11]. As a colonoscopic metric for tumor size, the Tumor Size Index (TSI) was calculated as (tumor area/colonic lumen area)×100 (%). Tumor-bearing mice were randomly assigned to treatment with either control vehicle alone (n = 8) or 45 mg/kg body weight Dactolisib (BEZ235, NVP-BEZ 235) in 10% 1-methyl-2-pyrrolidone/90% PEG 300 (n = 8) by daily oral gavage for 28 days. The treatment dose was chosen based on literature indicating that 40–50 mg/kg body weight Dactolisib (BEZ235, NVP-BEZ 235) effectively treats murine tumor models without adverse effects. Based on pharmacokinetic studies demonstrating maximal tissue concentration one hour after NVP-BEZ235 administration, tumor-bearing mice were sacrificed one hour after final treatment dose. Colonic tumor volume was assessed using calipers (width×length×height) and tumors were harvested for both western blot analysis and immunohistochemistry. |
| ADME/Pharmacokinetics |
The pharmacokinetic properties of NVP-BEZ235 were originally evaluated in PC3M tumor-bearing nude mice. At a dose of 50 mg/kg, NVP-BEZ235 appeared rapidly in plasma with a Cmax of 1.68 μmol/L at 0.5 h and a C24h of 0.03 μmol/L. In the tumor tissue, the Cmax attained was 2.05 nmol/g at 1 h (Tmax), which decreased to 0.23 nmol/g after 24 h (Fig. 5A). NVP-BEZ235 is eliminated relatively quickly from the liver. In vivo analysis of the S473P-Akt levels in the tumor tissue revealed that maximum inhibition was obtained 1 h after dosing (corresponding to the tumor Cmax), and persistent inhibition was observed still 16 h after treatment, with almost complete recovery to basal levels obtained in two of four tumors at 24 h after dose (Fig. 5B). Pharmacokinetic simulation based on this study revealed that steady-state levels would be achieved between 3 and 5 days, when dosing either at 50 mg/kg given daily or at 25 mg/kg given twice daily. Differences between the dosage regimens would reside in the predicted tumor peak levels (2.6 versus 1.6 nmol/g, respectively) whereas through levels would remain almost comparable (0.53 versus 0.60 μmol/L, respectively). Taking into consideration this pharmacokinetic simulation, chronic treatment of PC3M tumor-bearing animals was done at 25 mg/kg p.o. NVP-BEZ235 twice daily. Using this schedule, a statistically significant inhibition of tumor growth was observed, with a final T/C value of 22% after 10 days of treatment (Fig. 5C). The treatment was well tolerated as concluded from the nonstatistically significant effect of NVP-BEZ235 on body weight gain (Fig. 5C) and by the fact that none of the animals died during the course of the study. The antitumor effect was well correlated with the inhibition of S473P-Akt in the tumor tissue 1 or 18 h after the last dose detected either by Western blotting of tumor extracts or by immunostaining of tumor sections (Fig. 5D). Compound concentrations at these time points were 1.32 nmol/g at 1 h and 0.51 nmol/g at 18 h, close to the predicted values for steady-state peak levels (see above).[1]
|
| References |
|
| Additional Infomation |
Dactolisib is an imidazoquinoline that is 3-methyl-2-oxo-2,3-dihydro-1H-imidazo[4,5-c]quinoline substituted at position 1 by a 4-(1-cyanoisopropyl)phenyl group and at position 8 by a quinolin-3-yl group. A dual PI3K/mTOR inhibitor used in cancer treatment. It has a role as an EC 2.7.1.137 (phosphatidylinositol 3-kinase) inhibitor, a mTOR inhibitor and an antineoplastic agent. It is an imidazoquinoline, a nitrile, a member of quinolines, a ring assembly and a member of ureas.
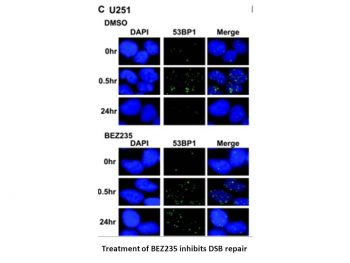
Dactolisib has been used in trials studying the treatment of Cancer, Solid Tumor, Renal Cancer, Breast Cancer, and Cowden Syndrome, among others. Dactolisib is an orally bioavailable imidazoquinoline targeting the phosphatidylinositol 3 kinase (PI3K) and the mammalian target of rapamycin (mTOR), with potential antineoplastic activity. Dactolisib inhibits PI3K kinase and mTOR kinase in the PI3K/AKT/mTOR kinase signaling pathway, which may result in tumor cell apoptosis and growth inhibition in PI3K/mTOR-overexpressing tumor cells. Activation of the PI3K/mTOR pathway promotes cell growth, survival, and resistance to chemotherapy and radiotherapy; mTOR, a serine/threonine kinase downstream of PI3K, may also be activated independent of PI3K. The phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin inhibitor (mTOR) pathway is often constitutively activated in human tumor cells, providing unique opportunities for anticancer therapeutic intervention. NVP-BEZ235 is an imidazo[4,5-c]quinoline derivative that inhibits PI3K and mTOR kinase activity by binding to the ATP-binding cleft of these enzymes. In cellular settings using human tumor cell lines, this molecule is able to effectively and specifically block the dysfunctional activation of the PI3K pathway, inducing G(1) arrest. The cellular activity of NVP-BEZ235 translates well in in vivo models of human cancer. Thus, the compound was well tolerated, displayed disease stasis when administered orally, and enhanced the efficacy of other anticancer agents when used in in vivo combination studies. Ex vivo pharmacokinetic/pharmacodynamic analyses of tumor tissues showed a time-dependent correlation between compound concentration and PI3K/Akt pathway inhibition. Collectively, the preclinical data show that NVP-BEZ235 is a potent dual PI3K/mTOR modulator with favorable pharmaceutical properties. NVP-BEZ235 is currently in phase I clinical trials.[1] Purpose: To examine the in vitro and in vivo efficacy of the dual PI3K/mTOR inhibitor NVP-BEZ235 in treatment of PIK3CA wild-type colorectal cancer (CRC). Experimental design: PIK3CA mutant and wild-type human CRC cell lines were treated in vitro with NVP-BEZ235, and the resulting effects on proliferation, apoptosis, and signaling were assessed. Colonic tumors from a genetically engineered mouse (GEM) model for sporadic wild-type PIK3CA CRC were treated in vivo with NVP-BEZ235. The resulting effects on macroscopic tumor growth/regression, proliferation, apoptosis, angiogenesis, and signaling were examined. Results: In vitro treatment of CRC cell lines with NVP-BEZ235 resulted in transient PI3K blockade, sustained decreases in mTORC1/mTORC2 signaling, and a corresponding decrease in cell viability (median IC(50) = 9.0-14.3 nM). Similar effects were seen in paired isogenic CRC cell lines that differed only in the presence or absence of an activating PIK3CA mutant allele. In vivo treatment of colonic tumor-bearing mice with NVP-BEZ235 resulted in transient PI3K inhibition and sustained blockade of mTORC1/mTORC2 signaling. Longitudinal tumor surveillance by optical colonoscopy demonstrated a 97% increase in tumor size in control mice (p = 0.01) vs. a 43% decrease (p = 0.008) in treated mice. Ex vivo analysis of the NVP-BEZ235-treated tumors demonstrated a 56% decrease in proliferation (p = 0.003), no effects on apoptosis, and a 75% reduction in angiogenesis (p = 0.013). Conclusions: These studies provide the preclinical rationale for studies examining the efficacy of the dual PI3K/mTOR inhibitor NVP-BEZ235 in treatment of PIK3CA wild-type CRC.[2] |
| Molecular Formula |
C30H23N5O
|
|---|---|
| Molecular Weight |
469.5365
|
| Exact Mass |
469.19026
|
| Elemental Analysis |
C, 76.74; H, 4.94; N, 14.92; O, 3.41
|
| CAS # |
915019-65-7
|
| Related CAS # |
Dactolisib Tosylate;1028385-32-1; 915019-65-7; 2319647-83-9 (HCl)
|
| PubChem CID |
11977753
|
| Appearance |
White to light yellow solid powder
|
| Density |
1.3±0.1 g/cm3
|
| Boiling Point |
701.0±70.0 °C at 760 mmHg
|
| Melting Point |
288-289°C
|
| Flash Point |
377.8±35.7 °C
|
| Vapour Pressure |
0.0±2.2 mmHg at 25°C
|
| Index of Refraction |
1.705
|
| LogP |
3.72
|
| Hydrogen Bond Donor Count |
0
|
| Hydrogen Bond Acceptor Count |
4
|
| Rotatable Bond Count |
3
|
| Heavy Atom Count |
36
|
| Complexity |
872
|
| Defined Atom Stereocenter Count |
0
|
| SMILES |
N#CC(C)(C)C1C=CC(N2C3C(=CN=C4C=3C=C(C3C=C5C(C=CC=C5)=NC=3)C=C4)N(C)C2=O)=CC=1
|
| InChi Key |
JOGKUKXHTYWRGZ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3
|
| Chemical Name |
2-Methyl-2-[4-[3-methyl-2-oxo-8-(quinolin-3-yl)-2,3-dihydroimidazo[4,5-c]quinolin-1-yl]phenyl]propionitrile.
|
| Synonyms |
BEZ 235; BEZ235; BEZ-235; Dactolisib; NVPBEZ235; NVP-BEZ235; BEZ235; NVP-BEZ 235; 2-methyl-2-(4-(3-methyl-2-oxo-8-(quinolin-3-yl)-2,3-dihydro-1H-imidazo[4,5-c]quinolin-1-yl)phenyl)propanenitrile; NVP-BEZ 235; NVP-BEZ-235; NVP-BEZ235
|
| HS Tariff Code |
2934.99.9001
|
| Storage |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| Shipping Condition |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| Solubility (In Vitro) |
DMSO: ~0.01 mg/mL (0.02 mM)
Water: <1 mg/mL (slightly soluble or insoluble) DMF: 18 mg/mL warming (38.33 mM) |
|---|---|
| Solubility (In Vivo) |
Solubility in Formulation 1: ≥ 0.52 mg/mL (1.11 mM) (saturation unknown) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (add these co-solvents sequentially from left to right, and one by one), clear solution.
For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 5.2 mg/mL clear DMSO stock solution to 400 μL PEG300 and mix evenly; then add 50 μL Tween-80 to the above solution and mix evenly; then add 450 μL normal saline to adjust the volume to 1 mL. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 2: ≥ 0.52 mg/mL (1.11 mM) (saturation unknown) in 10% DMSO + 90% Corn Oil (add these co-solvents sequentially from left to right, and one by one), clear solution. For example, if 1 mL of working solution is to be prepared, you can add 100 μL of 5.2 mg/mL clear DMSO stock solution to 900 μL of corn oil and mix evenly. View More
Solubility in Formulation 3: NMP+polyethylene glycol 300 (10/90, v/v): 30mg/mL Solubility in Formulation 4: 12.5 mg/mL (26.62 mM) in 50% PEG300 50% Saline (add these co-solvents sequentially from left to right, and one by one), suspension solution; with ultrasonication. Preparation of saline: Dissolve 0.9 g of sodium chloride in 100 mL ddH₂ O to obtain a clear solution. Solubility in Formulation 5: ≥ 1.1 mg/mL (2.34 mM) (saturation unknown) in 10% 1-Methyl-2-pyrrolidinone 90% PEG300 (add these co-solvents sequentially from left to right, and one by one), clear solution. |
| Preparing Stock Solutions | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1297 mL | 10.6487 mL | 21.2974 mL | |
| 5 mM | 0.4259 mL | 2.1297 mL | 4.2595 mL | |
| 10 mM | 0.2130 mL | 1.0649 mL | 2.1297 mL |
*Note: Please select an appropriate solvent for the preparation of stock solution based on your experiment needs. For most products, DMSO can be used for preparing stock solutions (e.g. 5 mM, 10 mM, or 20 mM concentration); some products with high aqueous solubility may be dissolved in water directly. Solubility information is available at the above Solubility Data section. Once the stock solution is prepared, aliquot it to routine usage volumes and store at -20°C or -80°C. Avoid repeated freeze and thaw cycles.
Calculation results
Working concentration: mg/mL;
Method for preparing DMSO stock solution: mg drug pre-dissolved in μL DMSO (stock solution concentration mg/mL). Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug.
Method for preparing in vivo formulation::Take μL DMSO stock solution, next add μL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O,mix and clarify.
(1) Please be sure that the solution is clear before the addition of next solvent. Dissolution methods like vortex, ultrasound or warming and heat may be used to aid dissolving.
(2) Be sure to add the solvent(s) in order.
| NCT Number | Status | Interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03373903 | Active Recruiting |
Drug: BEZ235 Drug: BEZ235 plus everolimus (RAD001) |
Respiratory Tract Infections | Restorbio Inc. | November 15, 2017 | Phase 2 |
| NCT04584710 | Active Recruiting |
Drug: RTB101 Drug: Placebo |
Covid19 | Restorbio Inc. | October 13, 2020 | Phase 2 |
Effect of the dual PI3K/mTOR inhibitor NVP-BEZ235 on 3D organotypic cultures of rat primary NFPA cells. |
Expression of DEFB1 in human NFPAs and immortalized gonadotroph cells. Clin Cancer Res. 2015 Jul 15;21(14):3204-15. |
Effect of the dual PI3K/mTOR inhibitor NVP-BEZ235 in vivo. |
Expression ofDEFB1in human NFPAs and immortalized gonadotroph cells.Clin Cancer Res.2015 Jul 15;21(14):3204-15. |
Role ofDEFB1in NET cell lines.Clin Cancer Res.2015 Jul 15;21(14):3204-15. |
Expression ofDefb1,Tnfrsf10b, andBcl2a1in rat pituitary adenoma tissues after NVP-BEZ235 treatmentin vivo.Clin Cancer Res.2015 Jul 15;21(14):3204-15. |
|
 |
 |